- Record: found
- Abstract: found
- Article: found
Identification of Vaccinia Virus Inhibitors and Cellular Functions Necessary for Efficient Viral Replication by Screening Bioactives and FDA-Approved Drugs
Read this article at
Abstract
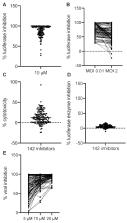
Four decades after the eradication of smallpox, poxviruses continue to threaten the health of humans and other animals. Vaccinia virus (VACV) was used as the vaccine that successfully eradicated smallpox and is a prototypic member of the poxvirus family. Many cellular pathways play critical roles in productive poxvirus replication. These pathways provide opportunities to expand the arsenal of poxvirus antiviral development by targeting the cellular functions required for efficient poxvirus replication. In this study, we developed and optimized a secreted Gaussia luciferase-based, simplified assay procedure suitable for high throughput screening. Using this procedure, we screened a customized compound library that contained over 3200 bioactives and FDA (Food and Drug Administration)-approved chemicals, most having known cellular targets, for their inhibitory effects on VACV replication. We identified over 140 compounds that suppressed VACV replication. Many of these hits target cellular pathways previously reported to be required for efficient VACV replication, validating the effectiveness of our screening. Importantly, we also identified hits that target cellular functions with previously unknown roles in the VACV replication cycle. Among those in the latter category, we verified the antiviral role of several compounds targeting the janus kinase/signal transducer and activator of transcription-3 (JAK/STAT3) signaling pathway by showing that STAT3 inhibitors reduced VACV replication. Our findings identify pathways that are candidates for use in the prevention and treatment of poxvirus infections and additionally provide a foundation to investigate diverse cellular pathways for their roles in poxvirus replications.
Related collections
Most cited references82
- Record: found
- Abstract: found
- Article: not found
mTOR at the nexus of nutrition, growth, ageing and disease

- Record: found
- Abstract: found
- Article: found
A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy
- Record: found
- Abstract: found
- Article: not found