- Record: found
- Abstract: found
- Article: found
Individual‐specific variation in the respiratory activities of HMECs and their bioenergetic response to IGF1 and TNFα
Read this article at
Abstract
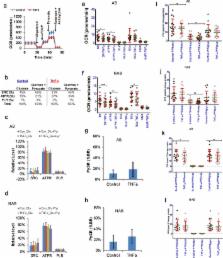
Metabolic reprograming is a hallmark of cancer cells. However, the roles of pre‐existing differences in normal cells metabolism toward cancer risk is not known. In order to assess pre‐existing variations in normal cell metabolism, we have quantified the inter‐individual variation in oxidative metabolism of normal primary human mammary epithelial cells (HMECs). We then assessed their response to selected cytokines such as insulin growth factor 1 (IGF1) and tumor necrosis factor alpha (TNFα), which are associated with breast cancer risk. Specifically, we compared the oxidative metabolism of HMECs obtained from women with breast cancer and without cancer. Our data show considerable inter‐individual variation in respiratory activities of HMECs from different women. A bioenergetic parameter called pyruvate‐stimulated respiration (PySR) was identified as a key distinguishing feature of HMECs from women with breast cancer and without cancer. Samples showing PySR over 20% of basal respiration rate were considered PySR +ve and the rest as PySR −ve. By this criterion, HMECs from tumor‐affected breasts (AB) and non‐tumor affected breasts (NAB) of cancer patients were mostly PySR −ve (88% and 89%, respectively), while HMECs from non‐cancer patients were mostly PySR +ve (57%). This suggests that PySR −ve/+ve phenotypes are individual‐specific and are not caused by field effects due to the presence of tumor. The effects of IGF1 and TNFα treatments on HMECs revealed that both suppressed respiration and extracellular acidification. In addition, IGF1 altered PySR −ve/+ve phenotypes. These results reveal individual‐specific differences in pyruvate metabolism of normal breast epithelial cells and its association with breast cancer risk.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: found
Glucose-Independent Glutamine Metabolism via TCA Cycling for Proliferation and Survival in B Cells
- Record: found
- Abstract: found
- Article: not found
A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans.
- Record: found
- Abstract: found
- Article: not found