- Record: found
- Abstract: found
- Article: found
S-Allylmercaptocysteine Targets Nrf2 in Osteoarthritis Treatment Through NOX4/NF-κB Pathway
Abstract
Purpose
This study aimed to explore the potential role and mechanism of garlic-derived S-allylmercaptocysteine (SAMC), the major water-soluble fraction of garlic, in osteoarthritis (OA) both in vivo and in vitro.
Methods
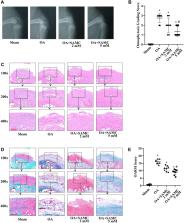
The effect of SAMC in a surgical-induced OA model was examined by X-ray, staining, ELISA, and immunoblotting. Then the key role of Nrf2 by SAMC treatment in IL-1β stimulated chondrocytes in vitro was determined by gene-knockdown technique.
Results
SAMC could stabilize the extracellular matrix (ECM) by decreasing metalloproteinase (MMPs) expression to suppress type II collagen degradation in OA rats. The inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, were elevated in OA, which could be down-regulated by SAMC treatment. This effect was parallel with NF-κB signaling inhibition by SAMC. As oxidative stress has been shown to participate in the inflammatory pathways in OA conditions, the key regulator Nrf2 in redox-homeostasis was evaluated in SAMC-treated OA rats. Nrf2 and its down-stream gene NQO-1 were activated in the SAMC-treated group, accompanied by NAD(P)H oxidases 4 (NOX4) expression down-regulated. As a result, the toxic lipid peroxidation byproduct 4-hydroxynonenal (4HNE) was reduced in articular cartilage. In IL-1β-stimulated primary rat chondrocytes, which could mimic OA in vitro, SAMC could ameliorate collagen destruction, inhibit inflammation, and maintain redox-homeostasis. Interestingly, after Nrf2 gene knockdown by adenovirus, the protective effect of SAMC in IL-1β-stimulated chondrocytes disappeared.
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Nrf2 signaling pathway: Pivotal roles in inflammation.
- Record: found
- Abstract: found
- Article: not found
Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance.
- Record: found
- Abstract: found
- Article: not found