- Record: found
- Abstract: found
- Article: found
Type I interferon is required for T helper (Th) 2 induction by dendritic cells
Read this article at
Abstract
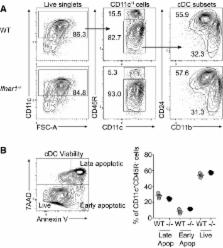
Type 2 inflammation is a defining feature of infection with parasitic worms (helminths), as well as being responsible for widespread suffering in allergies. However, the precise mechanisms involved in T helper (Th) 2 polarization by dendritic cells ( DCs) are currently unclear. We have identified a previously unrecognized role for type I IFN ( IFN‐I) in enabling this process. An IFN‐I signature was evident in DCs responding to the helminth Schistosoma mansoni or the allergen house dust mite ( HDM). Further, IFN‐I signaling was required for optimal DC phenotypic activation in response to helminth antigen (Ag), and efficient migration to, and localization with, T cells in the draining lymph node ( dLN). Importantly, DCs generated from Ifnar1 −/− mice were incapable of initiating Th2 responses in vivo. These data demonstrate for the first time that the influence of IFN‐I is not limited to antiviral or bacterial settings but also has a central role to play in DC initiation of Th2 responses.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function.
- Record: found
- Abstract: found
- Article: not found
IFNalpha activates dormant haematopoietic stem cells in vivo.
- Record: found
- Abstract: found
- Article: not found