- Record: found
- Abstract: found
- Article: found
Management and Care of Women With Invasive Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Clinical Practice Guideline
Read this article at
Abstract
Purpose
To provide evidence-based, resource-stratified global recommendations to clinicians and policymakers on the management and palliative care of women diagnosed with invasive cervical cancer.
Methods
ASCO convened a multidisciplinary, multinational panel of cancer control, medical and radiation oncology, health economic, obstetric and gynecologic, and palliative care experts to produce recommendations reflecting resource-tiered settings. A systematic review of literature from 1966 to 2015 failed to yield sufficiently strong quality evidence to support basic- and limited-resource setting recommendations; a formal consensus-based process was used to develop recommendations. A modified ADAPTE process was also used to adapt recommendations from existing guidelines.
Results
Five existing sets of guidelines were identified and reviewed, and adapted recommendations form the evidence base. Eight systematic reviews, along with cost-effectiveness analyses, provided indirect evidence to inform the consensus process, which resulted in agreement of 75% or greater.
Recommendations
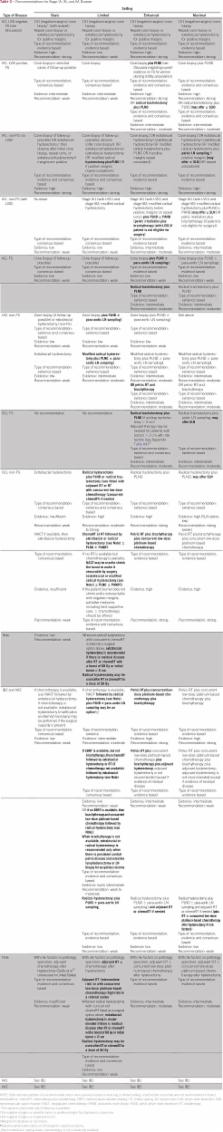
Clinicians and planners should strive to provide access to the most effective evidence-based antitumor and palliative care interventions. If a woman cannot access these within her own or neighboring country or region, she may need to be treated with lower-tier modalities, depending on capacity and resources for surgery, chemotherapy, radiation therapy, and supportive and palliative care. For women with early-stage cervical cancer in basic settings, cone biopsy or extrafascial hysterectomy may be performed. Fertility-sparing procedures or modified radical or radical hysterectomy may be additional options in nonbasic settings. Combinations of surgery, chemotherapy, and radiation therapy (including brachytherapy) should be used for women with stage IB to IVA disease, depending on available resources. Pain control is a vital component of palliative care. Additional information is available at www.asco.org/rs-cervical-cancer-treatment-guideline and www.asco.org/guidelineswiki. It is the view of ASCO that health care providers and health care system decision makers should be guided by the recommendations for the highest stratum of resources available. The guideline is intended to complement but not replace local guidelines.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Improved survival with bevacizumab in advanced cervical cancer.
- Record: found
- Abstract: found
- Article: not found
Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study.
- Record: found
- Abstract: found
- Article: not found