- Record: found
- Abstract: found
- Article: found
Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair
Read this article at
Abstract
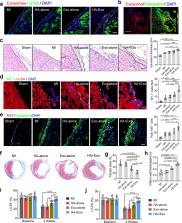
Cardiac patches are an effective way to deliver therapeutics to the heart. However, such procedures are normally invasive and difficult to perform. Here, we develop and test a method to utilize the pericardial cavity as a natural “mold” for in situ cardiac patch formation after intrapericardial injection of therapeutics in biocompatible hydrogels. In rodent models of myocardial infarction, we demonstrate that intrapericardial injection is an effective and safe method to deliver hydrogels containing induced pluripotent stem cells-derived cardiac progenitor cells or mesenchymal stem cells-derived exosomes. After injection, the hydrogels form a cardiac patch-like structure in the pericardial cavity, mitigating immune response and increasing the cardiac retention of the therapeutics. With robust cardiovascular repair and stimulation of epicardium-derived cells, the delivered therapeutics mitigate cardiac remodeling and improve cardiac functions post myocardial infarction. Furthermore, we demonstrate the feasibility of minimally-invasive intrapericardial injection in a clinically-relevant porcine model. Collectively, our study establishes intrapericardial injection as a safe and effective method to deliver therapeutic-bearing hydrogels to the heart for cardiac repair.
Abstract
Current routes to deliver therapeutics to the heart are normally invasive or difficult to perform. Here the authors develop intrapericardial injection as an efficient, easy-to-perform and minimally invasive method to deliver therapeutics for cardiac repair.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association
- Record: found
- Abstract: found
- Article: not found
Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice.
- Record: found
- Abstract: found
- Article: not found