- Record: found
- Abstract: found
- Article: found
Sensitive and Selective Detection of Tartrazine Based on TiO 2-Electrochemically Reduced Graphene Oxide Composite-Modified Electrodes

Read this article at
Abstract
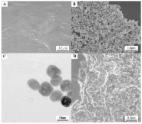
TiO 2-reduced graphene oxide composite-modified glassy carbon electrodes (TiO 2–ErGO–GCE) for the sensitive detection of tartrazine were prepared by drop casting followed by electrochemical reduction. The as-prepared material was characterized by transmission electron microscopy (TEM) and X-ray diffraction (XRD). Cyclic voltammetry and second-order derivative linear scan voltammetry were performed to analyze the electrochemical sensing of tartrazine on different electrodes. The determination conditions (including pH, accumulation potential, and accumulation time) were optimized systematically. The results showed that the TiO 2–ErGO composites increased the electrochemical active area of the electrode and enhanced the electrochemical responses to tartrazine significantly. Under the optimum detection conditions, the peak current was found to be linear for tartrazine concentrations in the range of 2.0 × 10 −8–2.0 × 10 −5 mol/L, with a lower detection limit of 8.0 × 10 −9 mol/L (S/N = 3). Finally, the proposed TiO 2–ErGO–GCEs were successfully applied for the detection of trace tartrazine in carbonated beverage samples.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Green Synthesis of Fluorescent Carbon Dots for Selective Detection of Tartrazine in Food Samples.
- Record: found
- Abstract: found
- Article: found
Fabrication of Amine-Modified Magnetite-Electrochemically Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode for Sensitive Dopamine Determination
- Record: found
- Abstract: not found
- Article: not found