- Record: found
- Abstract: found
- Article: found
Long-lived charge carrier generation in ordered films of a covalent perylenediimide–diketopyrrolopyrrole–perylenediimide molecule†
Read this article at
Abstract

Abstract
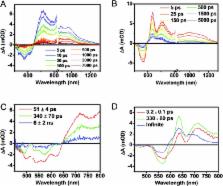
The photophysics of a covalently linked perylenediimide–diketopyrrolopyrrole–perylenediimide acceptor–donor–acceptor molecule (PDI–DPP–PDI, 1) were investigated and found to be markedly different in solution versus in unannealed and solvent annealed films. Photoexcitation of 1 in toluene results in quantitative charge separation in τ = 3.1 ± 0.2 ps, with charge recombination in τ = 340 ± 10 ps, while in unannealed/disordered films of 1, charge separation occurs in τ < 250 fs, while charge recombination displays a multiexponential decay in ∼6 ns. The absence of long-lived, charge separation in the disordered film suggests that few free charge carriers are generated. In contrast, upon CH 2Cl 2 vapor annealing films of 1, grazing-incidence X-ray scattering shows that the molecules form a more ordered structure. Photoexcitation of the ordered films results in initial formation of a spin-correlated radical ion pair (electron–hole pair) as indicated by magnetic field effects on the formation of free charge carriers which live for ∼4 μs. This result has significant implications for the design of organic solar cells based on covalent donor–acceptor systems and shows that long-lived, charge-separated states can be achieved by controlling intramolecular charge separation dynamics in well-ordered systems.
Related collections
Most cited references119
- Record: found
- Abstract: not found
- Article: not found
Bulk heterojunction solar cells with internal quantum efficiency approaching 100%
- Record: found
- Abstract: found
- Article: not found
Rylene and related diimides for organic electronics.
Author and article information
Notes
†Electronic supplementary information (ESI) available: Experimental details including synthetic methods, electrochemical characterization, and spectroscopic characterization of molecules 2 and 3 are given as described in the text. See DOI: 10.1039/c4sc02551b