- Record: found
- Abstract: found
- Article: not found
Drosophila phosphopantothenoylcysteine synthetase is required for tissue morphogenesis during oogenesis
Read this article at
Abstract
Background
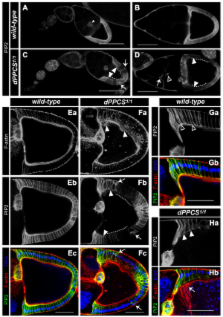
Coenzyme A (CoA) is an essential metabolite, synthesized from vitamin B 5 by the subsequent action of five enzymes: PANK, PPCS, PPCDC, PPAT and DPCK. Mutations in Drosophila dPPCS disrupt female fecundity and in this study we analyzed the female sterile phenotype of dPPCS mutants in detail.
Results
We demonstrate that dPPCS is required for various processes that occur during oogenesis including chorion patterning. Our analysis demonstrates that a mutation in dPPCS disrupts the organization of the somatic and germ line cells, affects F-actin organization and results in abnormal PtdIns(4,5)P 2 localization. Improper cell organization coincides with aberrant localization of the membrane molecules Gurken (Grk) and Notch, whose activities are required for specification of the follicle cells that pattern the eggshell. Mutations in dPPCS also induce alterations in scutellar patterning and cause wing vein abnormalities. Interestingly, mutations in dPANK and dPPAT-DPCK result in similar patterning defects.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis.
- Record: found
- Abstract: found
- Article: not found
Mass transit: epithelial morphogenesis in the Drosophila egg chamber.
- Record: found
- Abstract: found
- Article: not found
