- Record: found
- Abstract: found
- Article: not found
Ultrafast and high-throughput mass spectrometric assay for therapeutic drug monitoring of antiretroviral drugs in pediatric HIV-1 infection applying dried blood spots
Read this article at
Abstract
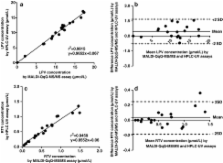
Kaletra® (Abott Laboratories) is a co-formulated medication used in the treatment of HIV-1-infected children, and it contains the two antiretroviral protease inhibitor drugs lopinavir and ritonavir. We validated two new ultrafast and high-throughput mass spectrometric assays to be used for therapeutic drug monitoring of lopinavir and ritonavir concentrations in whole blood and in plasma from HIV-1-infected children. Whole blood was blotted onto dried blood spot (DBS) collecting cards, and plasma was collected simultaneously. DBS collecting cards were extracted by an acetonitrile/water mixture while plasma samples were deproteinized with acetone. Drug concentrations were determined by matrix-assisted laser desorption/ionization-triple quadrupole tandem mass spectrometry (MALDI-QqQ-MS/MS). The application of DBS made it possible to measure lopinavir and ritonavir in whole blood in therapeutically relevant concentrations. The MALDI-QqQ-MS/MS plasma assay was successfully cross-validated with a commonly used high-performance liquid chromatography (HPLC)–ultraviolet (UV) assay for the therapeutic drug monitoring (TDM) of HIV-1-infected patients, and it showed comparable performance characteristics. Observed DBS concentrations showed as well, a good correlation between plasma concentrations obtained by MALDI-QqQ-MS/MS and those obtained by the HPLC-UV assay. Application of DBS for TDM proved to be a good alternative to the normally used plasma screening. Moreover, collection of DBS requires small amounts of whole blood which can be easily performed especially in (very) young children where collection of large whole blood amounts is often not possible. DBS is perfectly suited for TDM of HIV-1-infected children; but nevertheless, DBS can also easily be applied for TDM of patients in areas with limited or no laboratory facilities.
Related collections
Most cited references25
- Record: found
- Abstract: not found
- Article: not found
Measurement in Medicine: The Analysis of Method Comparison Studies
- Record: found
- Abstract: found
- Article: not found
