- Record: found
- Abstract: found
- Article: found
Increased Expression of CCN2, Epithelial Membrane Antigen, and Fibroblast Activation Protein in Hepatocellular Carcinoma with Fibrous Stroma Showing Aggressive Behavior
Read this article at
Abstract
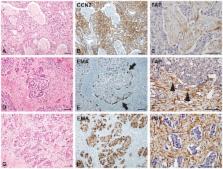
Tumor behavior is affected by the tumor microenvironment, composed of cancer-associated fibroblasts (CAFs). Meanwhile, hepatocellular carcinomas (HCC) with fibrous stroma reportedly exhibit aggressive behavior suggestive of tumor-stroma interaction. However, evidence of the crosstalk remains unclear. In this study, CCN2, epithelial membrane antigen (EMA), fibroblast activation protein (FAP), and keratin 19 (K19) expression was studied in 314 HCCs (cohort 1), 42 scirrhous HCCs (cohort 2), and 36 chronic hepatitis/cirrhosis specimens by immunohistochemistry. Clinicopathological parameters were analyzed according to the expressions of these markers. In tumor epithelial cells from cohort 1, CCN2 and EMA were expressed in 15.3% and 17.2%, respectively, and their expressions were more frequent in HCCs with fibrous stroma (≥5% of tumor area) than those without (P<0.05 for all); CCN2 expression was well correlated with K19 and EMA expression. In tumor stromal cells, FAP expression was found in 6.7%. In cohort 2, CCN2, EMA, and FAP expression was noted in 40.5%, 40.5%, and 66.7%, respectively, which was more frequent than that in cohort 1 (P<0.05 for all). Additionally, EMA expression was associated with the expression of K19, CCN2, and FAP (P<0.05 for all); EMA expressing tumor epithelial cells showed a topographic closeness to FAP-expressing CAFs. Analysis of disease-free survival revealed CCN2 expression to be a worse prognostic factor in both cohort 1 ( P = 0.005) and cohort 2 ( P = 0.023), as well as EMA as a worse prognostic factor in cohort 2 ( P = 0.048). In conclusion, expression of CCN2, EMA, and FAP may be involved in the activation of CAFs in HCC, giving rise to aggressive behavior. Significant correlation between EMA-expressing tumor cells and FAP-expressing CAFs and their topographic closeness suggests possible cross-talk between tumor epithelial cells and stromal cells in the tumor microenvironment of HCC.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: found
Basement membranes: structure, assembly and role in tumour angiogenesis.
- Record: found
- Abstract: found
- Article: not found
Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance.
- Record: found
- Abstract: found
- Article: not found