- Record: found
- Abstract: found
- Article: found
Treatment of grass pollen allergy: focus on a standardized grass allergen extract – Grazax ®
Read this article at
Abstract
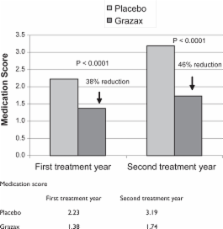
Immunotherapy is the only treatment for allergy that has the potential to alter the natural course of the disease. Sublingual immunotherapy (SLIT) for grass pollen-induced rhino-conjunctivitis has been developed to make immunotherapy available to a broader group of allergic patients. In the largest clinical programme ever conducted with allergen-specific immunotherapy, over 1,700 adults and 260 children have been exposed to Grazax ®. Grazax is formulated as an oral lyophilisate (tablet) for sublingual administration, containing 75,000 SQ-T standardized allergen extract of grass pollen from Phleum pratense. Grazax is indicated for treatment of grass pollen-induced rhinitis and conjunctivitis in adult patients with clinically relevant symptoms and diagnosed with a positive skin prick test and/or specific IgE test to grass pollen. In phase I trials doses from 2,500 to 1,000,000 SQ-T were tested. All doses were well tolerated and 75,000 SQ-T, with approximately 15 μg major allergen protein, was chosen as the optimal dose. Three phase III trials are ongoing, one being a long-term trial. Results from GT-08 trial first and second treatment years showed a reduction of 30% and 36%, respectively, in daily rhino-conjunctivitis symptom scores and a reduction of 38% and 46% of daily rhino-conjunctivitis medication scores compared with placebo over the entire grass pollen season. Subjects treated with Grazax also had an increased number of well days and improved quality of life, and more subjects experienced excellent rhino-conjunctivitis control. The most common adverse events related to Grazax are local reactions, such as pruritus, edema mouth, ear pruritus, throat irritation, and sneezing. We conclude that Grazax is efficacious and safe for treatment of rhino-conjunctivitis due to grass pollen allergy.