- Record: found
- Abstract: found
- Article: found
Cross-Sectional Inverse Associations of Obesity and Fat Accumulation Indicators with Testosterone in Non-Diabetic Aging Men
Read this article at
Abstract
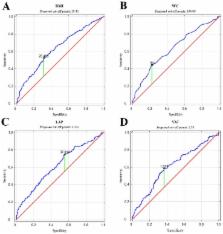
Introduction/Objective: The aim of the study was to show which of the adipose tissue accumulation indicators correlate with testosterone disorders in non-diabetic aging men. Material and methods: 455 non diabetic men, recruited at primary care facilities, aged 50–75 participated in the study. The participants underwent anthropometric measurement and ELISA determination of total testosterone (TT), estradiol (E 2), dehydroepiandrosterone sulphate (DHEA-S), sex hormone binding protein (SHBG), and the determination of fasting glucose (FPG), high-density lipids cholesterol (HDL-Ch), and triacylglycerols (TAG) in serum. The following indicators were calculated: body mass index (BMI), waist-to-hip ratio (WHR), lipid accumulation product (LAP), and visceral adiposity index (VAI). Results: Men with testosterone deficiency syndrome (TDS) differed in each of the assessed obesity indices from those without TDS. All of the studied parameters correlated significantly negatively with TT concentration in blood serum, with VAI being the strongest predictor of TDS. It was shown that the threshold value at which the risk of TDS increased was 28.41 kg/m 2 for BMI, 1.58 for VAI, 104 cm for WC, and 37.01 for LAP. Conclusions: Indicators of fat accumulation that take into account biochemical parameters in assessing lipid metabolism are better markers of actual body fat deposition than indicators based solely on anthropometric measurements. Among them, VAI seems the most suitable biomarker of TDS in non-diabetic aging men.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Testosterone: a vascular hormone in health and disease.
- Record: found
- Abstract: found
- Article: not found
The hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt--a major factor in the genesis of morbid obesity.
- Record: found
- Abstract: found
- Article: not found