- Record: found
- Abstract: found
- Article: found
Early Activation of STAT3 Regulates Reactive Astrogliosis Induced by Diverse Forms of Neurotoxicity
Read this article at
Abstract
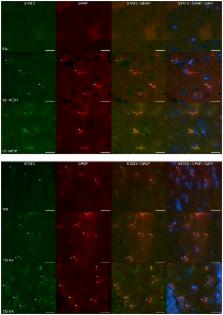
Astrogliosis, a cellular response characterized by astrocytic hypertrophy and accumulation of GFAP, is a hallmark of all types of central nervous system (CNS) injuries. Potential signaling mechanisms driving the conversion of astrocytes into “reactive” phenotypes differ with respect to the injury models employed and can be complicated by factors such as disruption of the blood-brain barrier (BBB). As denervation tools, neurotoxicants have the advantage of selective targeting of brain regions and cell types, often with sparing of the BBB. Previously, we found that neuroinflammation and activation of the JAK2-STAT3 pathway in astrocytes precedes up regulation of GFAP in the MPTP mouse model of dopaminergic neurotoxicity. Here we show that multiple mechanistically distinct mouse models of neurotoxicity (MPTP, AMP, METH, MDA, MDMA, KA, TMT) engender the same neuroinflammatory and STAT3 activation responses in specific regions of the brain targeted by each neurotoxicant. The STAT3 effects seen for TMT in the mouse could be generalized to the rat, demonstrating cross-species validity for STAT3 activation. Pharmacological antagonists of the neurotoxic effects blocked neuroinflammatory responses, pSTAT3 tyr705 and GFAP induction, indicating that damage to neuronal targets instigated astrogliosis. Selective deletion of STAT3 from astrocytes in STAT3 conditional knockout mice markedly attenuated MPTP-induced astrogliosis. Monitoring STAT3 translocation in GFAP-positive cells indicated that effects of MPTP, METH and KA on pSTAT3 tyr705 were localized to astrocytes. These findings strongly implicate the STAT3 pathway in astrocytes as a broadly triggered signaling pathway for astrogliosis. We also observed, however, that the acute neuroinflammatory response to the known inflammogen, LPS, can activate STAT3 in CNS tissue without inducing classical signs of astrogliosis. Thus, acute phase neuroinflammatory responses and neurotoxicity-induced astrogliosis both signal through STAT3 but appear to do so through different modules, perhaps localized to different cell types.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration.
- Record: found
- Abstract: found
- Article: not found
The JAK-STAT signaling pathway: input and output integration.
- Record: found
- Abstract: found
- Article: not found