- Record: found
- Abstract: found
- Article: found
Th1‐type immune responses to Porphyromonas gingivalis antigens exacerbate angiotensin II‐dependent hypertension and vascular dysfunction
Read this article at
Abstract
Background and Purpose
Emerging evidence indicates that hypertension is mediated by immune mechanisms. We hypothesized that exposure to Porphyromonas gingivalis antigens, commonly encountered in periodontal disease, can enhance immune activation in hypertension and exacerbate the elevation in BP, vascular inflammation and vascular dysfunction.
Experimental Approach
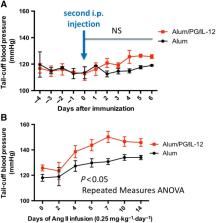
Th1 immune responses were elicited through immunizations using P. gingivalis lysate antigens (10 μg) conjugated with aluminium oxide (50 μg) and IL‐12 (1 μg). The hypertension and vascular endothelial dysfunction evoked by subpressor doses of angiotensin II (0.25 mg·kg −1·day −1) were studied, and vascular inflammation was quantified by flow cytometry and real‐time PCR.
Key Results
Systemic T‐cell activation, a characteristic of hypertension, was exacerbated by P. gingivalis antigen stimulation. This translated into increased aortic vascular inflammation with enhanced leukocyte, in particular, T‐cell and macrophage infiltration. The expression of the Th1 cytokines, IFN‐γ and TNF‐α, and the transcription factor, TBX21, was increased in aortas of P. gingivalis/IL‐12/aluminium oxide‐immunized mice, while IL‐4 and TGF‐β were unchanged. These immune changes in mice with induced T‐helper‐type 1 immune responses were associated with an enhanced elevation of BP and endothelial dysfunction compared with control mice in response to 2 week infusion of a subpressor dose of angiotensin II.
Conclusions and Implications
These results support the concept that Th1 immune responses induced by bacterial antigens such as P. gingivalis can increase sensitivity to subpressor pro‐hypertensive insults such as low‐dose angiotensin II, thus providing a mechanistic link between chronic infection, such as periodontitis, and hypertension.
Linked Articles
This article is part of a themed section on Immune Targets in Hypertension. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.12/issuetoc
Related collections
Most cited references44
- Record: found
- Abstract: not found
- Article: not found
Inflammation, immunity, and hypertension.
- Record: found
- Abstract: found
- Article: not found
Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension.

- Record: found
- Abstract: found
- Article: found