- Record: found
- Abstract: found
- Article: found
M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2

Read this article at
Abstract
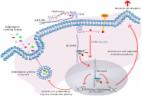
As a common female malignancy, triple-negative breast cancer (TNBC) is the most malignant subtype of breast cancers (BC). This study further studied the role of long noncoding RNA (lncRNA) prostate cancer-associated transcript 6 (PCAT6) in TNBC. Functional assays, including EdU, wound healing, transwell, and immunofluorescence staining, revealed the effect of PCAT6 on cell proliferation, migration, and EMT process. The tube-formation assay disclosed the function of PCAT6 on angiogenesis. In vivo assays were also established to explore the impact of PCAT6 on tumor growth and microangiogenesis. The results revealed that PCAT6 boosted TNBC cell proliferation, migration, and angiogenesis both in vitro and in vivo. Then, this study unveiled that M2 macrophage secreted VEGF to stimulate the upregulation of PCAT6, thus promoting angiogenesis in TNBC. Next, through bioinformatics analysis and mechanism assays, we identified that PCAT6 positively regulated VEGFR2 expression via ceRNA pattern and then participated in VEGFR/AKT/mTOR signaling pathway to accelerate angiogenesis. Moreover, PCAT6 bound USP14, a deubiquitinase, to induce the deubiquitination of VEGFR2. On the whole, M2 macrophage-induced upregulation of PCAT6 facilitates TNBC tumorigenesis through modulation of VEGFR2 expression via ceRNA and deubiquitination patterns.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer.
- Record: found
- Abstract: found
- Article: not found
Macrophage Polarization: Anti-Cancer Strategies to Target Tumor-Associated Macrophage in Breast Cancer.