- Record: found
- Abstract: found
- Article: not found
Development and Statistical Optimisation of Buspirone Hydrochloride Buccoadhesive Films
Read this article at
Abstract
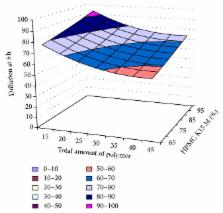
The aim of the present study was to prepare unidirectional buccal films of buspirone hydrochloride by solvent casting technique. Hydroxypropylmethylcellulose (HPMC K15M) and Eudragit RL-100 were used as polymers in different proportion. Polyethylene glycol 400 and sodium lauryl sulphate were used as plasticizer and permeation enhancer, respectively, in different concentration. In the formulation, total amount of polymer ( X 1) and percentage of HPMC K15M ( X 2) were kept as independent variables. Afterwards, statistically optimized process was carried out and two optimized formulations (OF1 and OF2) were developed. The observed results of optimized formulation were showed a greater degree of percentage of similarity with predicted values. The stability studies showed that there was no significant change found in physicochemical properties, in-vitro release, and ex-vivo diffusion studies.
Related collections
Most cited references16

- Record: found
- Abstract: found
- Article: found
Mucoadhesive drug delivery system: An overview
- Record: found
- Abstract: found
- Article: not found
Buccal mucosa as a route for systemic drug delivery: a review.
- Record: found
- Abstract: found
- Article: not found
