- Record: found
- Abstract: found
- Article: found
Proteomic analysis of human aqueous humor using multidimensional protein identification technology
Read this article at
Abstract
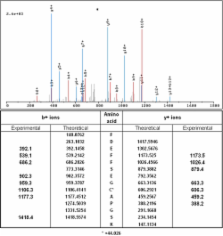
Aqueous humor (AH) supports avascular tissues in the anterior segment of the eye, maintains intraocular pressure, and potentially influences the pathogenesis of ocular diseases. Nevertheless, the AH proteome is still poorly defined despite several previous efforts, which were hindered by interfering high abundance proteins, inadequate animal models, and limited proteomic technologies. To facilitate future investigations into AH function, the AH proteome was extensively characterized using an advanced proteomic approach. Samples from patients undergoing cataract surgery were pooled and depleted of interfering abundant proteins and thereby divided into two fractions: albumin-bound and albumin-depleted. Multidimensional Protein Identification Technology (MudPIT) was utilized for each fraction; this incorporates strong cation exchange chromatography to reduce sample complexity before reversed-phase liquid chromatography and tandem mass spectrometric analysis. Twelve proteins had multi-peptide, high confidence identifications in the albumin-bound fraction and 50 proteins had multi-peptide, high confidence identifications in the albumin-depleted fraction. Gene ontological analyses were performed to determine which cellular components and functions were enriched. Many proteins were previously identified in the AH and for several their potential role in the AH has been investigated; however, the majority of identified proteins were novel and only speculative roles can be suggested. The AH was abundant in anti-oxidant and immunoregulatory proteins as well as anti-angiogenic proteins, which may be involved in maintaining the avascular tissues. This is the first known report to extensively characterize and describe the human AH proteome and lays the foundation for future work regarding its function in homeostatic and pathologic states.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
The human plasma proteome: a nonredundant list developed by combination of four separate sources.
- Record: found
- Abstract: not found
- Article: not found
The need for guidelines in publication of peptide and protein identification data: Working Group on Publication Guidelines for Peptide and Protein Identification Data.
- Record: found
- Abstract: found
- Article: not found