- Record: found
- Abstract: found
- Article: found
Mechanisms of melanocyte death in vitiligo
Read this article at
Abstract
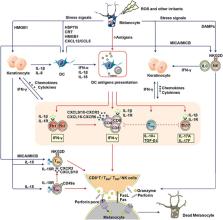
Vitiligo is an autoimmune depigment disease results from extensive melanocytes destruction. The destruction of melanocyte is thought to be of multifactorial causation. Genome‐wide associated studies have identified single‐nucleotide polymorphisms in a panel of susceptible loci as risk factors in melanocyte death. But vitiligo onset can't be solely attributed to a susceptive genetic background. Oxidative stress triggered by elevated levels of reactive oxygen species accounts for melanocytic molecular and organelle dysfunction, a minority of melanocyte demise, and melanocyte‐specific antigens exposure. Of note, the self‐responsive immune function directly contributes to the bulk of melanocyte deaths in vitiligo. The aberrantly heightened innate immunity, type‐1‐skewed T helper, and incompetent regulatory T cells tip the balance toward autoreaction and CD8 + cytotoxic T lymphocytes finally execute the killing of melanocytes, possibly alarmed by resident memory T cells. In addition to the well‐established apoptosis and necrosis, we discuss several death modalities like oxeiptosis, ferroptosis, and necroptosis that are probably employed in melanocyte destruction. This review focuses on the various mechanisms of melanocytic death in vitiligo pathogenesis to demonstrate a panorama of that. We hope to provide new insights into vitiligo pathogenesis and treatment strategies by the review.
Related collections
Most cited references247
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: an iron-dependent form of nonapoptotic cell death.
- Record: found
- Abstract: found
- Article: not found
Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease
- Record: found
- Abstract: found
- Article: not found