- Record: found
- Abstract: found
- Article: not found
Effect of Di-(2-ethylhexyl)-phthalate on Sphingolipid Metabolic Enzymes in Rat Liver
Read this article at
Abstract
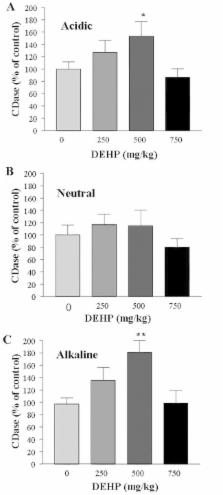
Di-(2-ethylhexyl)-phthalate (DEHP), the most widely utilized industrial plastizer and a ubiquitous environmental contaminant, can act on peroxisome proliferators-activated nuclear hormone receptor family (PPAR) isoforms. To understand the contribution of sphingolipid metabolism to DEHP-induced hepatotoxicity, effect of DEHP exposure on activities of sphingolipid metabolic enzymes in rat liver was investigated. DEHP (250, 500 or 750 mg/kg) was administered to the rats through oral gavage daily for 28 days. The activities of acidic and alkaline ceramidases were slightly increased in 250 mg/kg DEHP-administered rat livers and significantly elevated in 500 mg/kg DEHP-administered ones, although the level of 750 mg/kg DEHP-administered ones was not increased. Neutral ceramidase, acidic and neutral sphingomyelinases, sphingomyeline synthase and ceramide syhthase were not changed at all by DEHP exposure. Therefore, acidic and alkaline ceramidases might play important roles in DEHP-induced hepatotoxicity.
Related collections
Most cited references23
- Record: found
- Abstract: not found
- Article: not found
A rapid method of total lipid extraction and purification.
- Record: found
- Abstract: found
- Article: not found
Functions of ceramide in coordinating cellular responses to stress.
- Record: found
- Abstract: found
- Article: not found
