- Record: found
- Abstract: found
- Article: found
Role of the Neutrophil in the Pathogenesis of Advanced Cancer and Impaired Responsiveness to Therapy†
Read this article at
Abstract
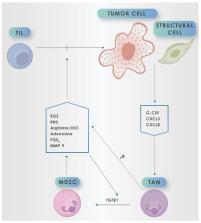
Notwithstanding the well-recognized involvement of chronic neutrophilic inflammation in the initiation phase of many types of epithelial cancers, a growing body of evidence has also implicated these cells in the pathogenesis of the later phases of cancer development, specifically progression and spread. In this setting, established tumors have a propensity to induce myelopoiesis and to recruit neutrophils to the tumor microenvironment (TME), where these cells undergo reprogramming and transitioning to myeloid-derived suppressor cells (MDSCs) with a pro-tumorigenic phenotype. In the TME, these MDSCs, via the production of a broad range of mediators, not only attenuate the anti-tumor activity of tumor-infiltrating lymphocytes, but also exclude these cells from the TME. Realization of the pro-tumorigenic activities of MDSCs of neutrophilic origin has resulted in the development of a range of adjunctive strategies targeting the recruitment of these cells and/or the harmful activities of their mediators of immunosuppression. Most of these are in the pre-clinical or very early clinical stages of evaluation. Notable exceptions, however, are several pharmacologic, allosteric inhibitors of neutrophil/MDSC CXCR1/2 receptors. These agents have entered late-stage clinical assessment as adjuncts to either chemotherapy or inhibitory immune checkpoint-targeted therapy in patients with various types of advanced malignancy. The current review updates the origins and identities of MDSCs of neutrophilic origin and their spectrum of immunosuppressive mediators, as well as current and pipeline MDSC-targeted strategies as potential adjuncts to cancer therapies. These sections are preceded by a consideration of the carcinogenic potential of neutrophils.
Related collections
Most cited references113
- Record: found
- Abstract: found
- Article: not found
The biological functions of T helper 17 cell effector cytokines in inflammation.
- Record: found
- Abstract: found
- Article: not found
Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps.
- Record: found
- Abstract: found
- Article: not found