- Record: found
- Abstract: found
- Article: found
Guideline of the Brazilian Society of Cardiology on Telemedicine in Cardiology - 2019
other
Marcelo Antônio Cartaxo Queiroga Lopes
1 ,
Gláucia Maria Moraes de Oliveira
2 ,
Antonio Luiz Pinho Ribeiro
3 ,
Fausto J. Pinto
4 ,
Helena Cramer Veiga Rey
5 ,
Leandro Ioschpe Zimerman
6 ,
Carlos Eduardo Rochitte
7 ,
Fernando Bacal
7 ,
Carisi Anne Polanczyk
6
,
8
,
9 ,
Cidio Halperin
10 ,
Edson Correia Araújo
11 ,
Evandro Tinoco Mesquita
12 ,
José Airton Arruda
13 ,
Luis Eduardo Paim Rohde
6 ,
Max Grinberg
7 ,
Miguel Moretti
7 ,
Paulo Ricardo Avancini Caramori
14 ,
Roberto Vieira Botelho
15
,
16 ,
Andréa Araújo Brandão
17 ,
Ludhmila Abrahão Hajjar
7 ,
Alexandre Fonseca Santos
11 ,
Alexandre Siciliano Colafranceschi
18 ,
Ana Paula Beck da Silva Etges
9 ,
Bárbara Campos Abreu Marino
19
,
20 ,
Bruna Stella Zanotto
8
,
9 ,
Bruno Ramos Nascimento
21 ,
Cesar Rocha Medeiros
22 ,
Daniel Vitor de Vasconcelos Santos
3 ,
Daniela Matos Arrowsmith Cook
18
,
23
,
24 ,
Eduardo Antoniolli
25 ,
Erito Marques de Souza Filho
12
,
26 ,
Fábio Fernandes
27 ,
Fabio Gandour
28 ,
Francisco Fernandez
16 ,
Germano Emilio Conceição Souza
29 ,
Guilherme de Souza Weigert
30
,
31 ,
Iran Castro
32
,
33 ,
Jamil Ribeiro Cade
34 ,
José Albuquerque de Figueiredo Neto
35 ,
Juliano de Lara Fernandes
36 ,
Marcelo Souza Hadlich
37
,
38
,
39 ,
Marco Antonio Praça Oliveira
29 ,
Maria Beatriz Alkmim
3
,
21 ,
Maria Cristina da Paixão
3 ,
Maurício Lopes Prudente
40 ,
Miguel A. S. Aguiar Netto
41 ,
Milena Soriano Marcolino
3 ,
Monica Amorim de Oliveira
5 ,
Osvaldo Simonelli
42
,
43 ,
Pedro A. Lemos Neto
44 ,
Priscila Raupp da Rosa
44
,
45 ,
Renato Minelli Figueira
3 ,
Roberto Caldeira Cury
46 ,
Rodrigo Coelho Almeida
47 ,
Sandra Regina Franco Lima
48 ,
Silvio Henrique Barberato
49
,
50 ,
Thiago Inocêncio Constancio
51 ,
Wladimir Fernandes de Rezende
52
November 2019
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Grades of recommendation and levels of evidence in this update were applied according
to the following standards:
Classes (grades) of recommendation
Grade I
Conditions for which there is conclusive evidence or, in the absence of conclusive
evidence, a general consensus that the procedure is safe and useful/effective.
Grade IIa
Conditions for which there is conflicting evidence and/or divergent opinions regarding
the procedure's safety and usefulness/effectiveness. Weight or evidence/opinion in
favor of the procedure. Received approval by most studies/experts.
Grade IIb
Conditions for which there is conflicting evidence and/or divergent opinions regarding
the procedure's safety and usefulness/effectiveness. Safety and usefulness/effectiveness
are less well established, with no prevailing opinions in favor.
Grade III
Conditions for which there is evidence and/or consensus that the procedure is not
useful/effective and in some cases may be potentially harmful.
Levels of evidence
Level A
Data obtained from multiple, concordant, large randomized trials, and/or robust meta-analysis
of randomized clinical trials.
Level B
Data obtained from less robust meta-analysis, from a single randomized trial, or from
nonrandomized (observational) trials.
Level C
Data obtained through a consensus of expert opinions.
Declaration of potential conflict of interests of authors/collaborators of theGuideline
of the Brazilian Society of Cardiology on Telemedicine in Cardiology - 2019If, within
the last 3 years, the author/collaborator of the guideline:
Names of guideline collaborators
Participated in clinical and/or experimental studies sponsored by pharmaceutical or
equipment companies related to this guideline
Spoke at events or activities sponsored by industry related to this guideline
Was (is) a member of a board of advisors or a board of directors of a pharmaceutical
or equipment industry
Participated in normative committees of scientific research sponsored by industry
Received personal or institutional funding from industry
Wrote scientific papers in journals sponsored by industry
Owns stocks in industry
Alexandre Fonseca Santos
No
No
No
No
No
No
No
Alexandre Siciliano Colafranceschi
No
No
No
No
No
No
No
Ana Paula Beck da Silva Etges
No
No
No
No
No
No
No
Andréa Araújo Brandão
No
No
No
No
No
No
No
Antonio Luiz Pinho Ribeiro
No
No
No
No
No
No
No
Bárbara Campos Abreu Marino
No
No
No
No
No
No
No
Bruna Stella Zanotto
No
No
No
No
No
No
No
Bruno Ramos Nascimento
No
No
No
No
No
No
No
Carisi Anne Polanczyk
No
No
No
No
No
No
No
Carlos Eduardo Rochitte
No
No
No
No
No
No
No
Cesar Rocha Medeiros
No
No
No
No
No
No
No
Cidio Halperin
Apple
No
No
No
No
No
No
Daniel Vitor de Vasconcelos Santos
No
No
No
No
No
No
No
Daniela Matos Arrowsmith Cook
No
No
No
No
No
No
No
Edson Correia Araújo
No
No
No
No
No
No
No
Eduardo Antoniolli
No
No
No
No
No
No
No
Erito Marques de Souza Filho
No
No
No
No
No
No
No
Evandro Tinoco Mesquita
No
No
No
No
No
No
No
Fábio Fernandes
No
No
No
No
No
No
No
Fabio Gandour
No
No
No
No
No
No
No
Fausto J. Pinto
No
No
No
No
No
No
No
Fernando Bacal
No
No
No
No
No
No
No
Francisco Fernandez
No
No
No
No
No
No
No
Germano Emilio Conceição Souza
No
No
No
No
No
No
No
Gláucia Maria Moraes de Oliveira
No
No
No
No
No
No
No
Guilherme de Souza Weigert
No
No
Conexa Saúde
No
Conexa Saúde
No
Conexa Saúde
Helena Cramer Veiga Rey
No
No
No
No
No
No
No
Iran Castro
No
No
No
No
No
No
No
Jamil Ribeiro Cade
No
No
No
No
No
No
No
José Airton de Arruda
No
No
No
No
No
No
No
José Albuquerque de Figueiredo Neto
No
No
No
No
No
No
No
Juliano Lara Fernandes
No
No
No
No
No
No
Hypera Pharma, Grupo Biotoscana
Leandro Ioschpe Zimerman
No
No
No
Pfizer
Bayer, Pfizer, Biotronik
No
No
Ludhmila Abrahão Hajjar
No
No
No
No
No
No
No
Luis Eduardo Paim Rohde
No
No
No
No
No
No
No
Marcelo Antônio Cartaxo Queiroga Lopes
No
No
No
No
No
No
No
Marcelo Souza Hadlich
No
No
No
No
No
No
No
Marco Antonio Praça Oliveira
No
No
No
No
No
No
No
Maria Beatriz Alkmim
No
No
No
No
No
No
No
Maria Cristina da Paixão
No
No
No
No
No
No
No
Maurício Lopes Prudente
No
No
No
No
No
No
No
Max Grinberg
No
No
No
No
No
No
No
Miguel A. S. Aguiar Netto
No
No
No
No
No
No
No
Miguel Antonio Moretti
No
No
No
No
No
No
No
Milena Soriano Marcolino
No
No
No
No
No
No
No
Monica Amorim de Oliveira
No
No
No
No
No
No
No
Osvaldo Simonelli
No
No
No
No
No
No
No
Paulo Ricardo Avancini Caramori
No
No
Medtronic
SciTech, Biotronik
No
No
No
Pedro A. Lemos Neto
No
No
No
No
No
No
No
Priscila Raupp da Rosa
No
Aruba/Kapersky
No
No
No
No
No
Renato Minelli Figueira
No
No
No
No
No
No
No
Roberto Caldeira Cury
No
No
No
No
No
No
No
Roberto Vieira Botelho
No
No
No
No
No
No
No
Rodrigo Coelho de Almeida
No
No
No
No
No
No
No
Sandra Regina Franco Lima
No
No
No
No
No
No
No
Silvio Henrique Barberato
No
No
No
No
No
No
No
Thiago Inocêncio Constancio
No
No
No
No
No
No
No
Wladimir Fernandes de Rezende
No
No
No
No
No
No
No
Presentation
In due time, the Brazilian Society of Cardiology decided to create a guideline on
telemedicine applied to cardiology, also known as telecardiology. According to the
Pan American Health Organization (PAHO) and the World Health Organization (WHO), telemedicine
is “The delivery of health care services, where distance is a critical factor, by
all health care professionals using information and communication technologies for
the exchange of valid information for diagnosis, treatment, and prevention of disease
and injuries, research and evaluation, and for the continuing education of health
care providers, all in the interests of advancing the health of individuals and their
communities.” Such a seemingly simple and altruistic definition carries a wide range
of potential implications at various levels, from an ethical point of view to a potential
impact on clinical practice and outcomes. Hence, the importance of guidelines, organized
by the medical community through scientific societies, in offering to all of those
involved in the process a reference based, as much as possible, on expert opinion,
current scientific evidence, and on respect for medical ethical and deontological
values.
Considering that cardiovascular diseases are the main cause of morbidity and mortality
in the 21st century in Brazil and worldwide, the opportunity to use instruments to
allow more effective actions in the prevention, diagnosis, treatment, and follow-up
of these diseases paves the way to very relevant perspectives of better care for the
populations and communities that we serve. At the same time, bioethical aspects and
consequences should never be neglected, as they can (and should) undermine programs
that, disguised as “medical,” fail to meet these ethical requirements. Therefore,
regulated operating models based on guidelines organized by medical-scientific authorities
are fundamental in striking a balance.
The introduction and implementation of new digital technologies are favoring the emergence
of new methodologies (many still experimental) aimed at improving the capacity of
intervention on individual patients and allowing for more customized care. We are
experiencing what Eric Topol
1
in his latest book, “Deep Medicine: How Artificial Intelligence Can Make Healthcare
Human Again,” called the “Fourth Industrial Age” comprising artificial intelligence,
robotics, and big data that will have a great impact on the way we live and see ourselves
as human beings. If this is very positive at first sight, it is also true that it
is not devoid of risk, particularly in the way that we approach or will approach the
patient. Therefore, one must not forget the Hippocratic principle: “It is far more
important to know what person the disease has than what disease the person has.” In
fact, when we are sick, we all want to have our doctor - and not a computer - taking
care of us and offering us a word of comfort and confidence.
Therefore, we must think smartly about how to apply to human benefit this impressive
array of elements that have opened up frontiers that were unfathomable just a few
years ago. Telemedicine - or telecardiology - can indeed play a very important role,
particularly when this may be the only available resource. However, its use must be
properly delineated to prevent abuse and misuse. The present document and guideline
was prepared for this purpose. This complete document offers a detailed review of
the regulation of telemedicine in Brazil, defines the meaning of a geographically
remote area, and describes the fundamentals of telemedicine and the secure grounds
for its transmission.
This document also offers up-to-date information on current evidence and applications
of so-called teleconsultation, telediagnosis, and telemonitoring, and reflects on
how telemedicine can provide technology-based medical services, with artificial intelligence
playing a key role. The document also includes the economic assessment and budgetary
impact of incorporating telemedicine in cardiology in Brazil and telemedicine in supplementary
health, and - in one of the most important chapters - presents the ethical and legal
aspects of telemedicine. Finally, the document includes a set of recommendations intended
to be practical and adapted to the Brazilian perspective.
The result is a guideline perfectly aligned with the WHO guidelines on the principle
that the implementation of telemedicine must be properly planned and should predict
situations like the feasibility of network coverage for technology access in remote
locations, construction of a legal and judicial structure for the implementation,
budgetary impact and cost-effectiveness assessment of the implementation of each stage
of the project, and development of indicators of the clinical continuum of applicability
for user safety. As the president-elect of the World Heart Federation, I see this
as a model document in terms of how it was planned and implemented, as well as in
its content, reflecting the current evidence and perspective of the main scientific
players in the area. As such, I think it will become a historical document, a milestone
in the responsible introduction of telemedicine-telecardiology in clinical practice,
in this case, applied to Brazil, but which can serve as an example for others globally,
contributing to decrease the burden of cardiovascular diseases worldwide.
Lisbon, June 2019.
Prof. Fausto J. Pinto, FESC, FACC
President-elect, World Heart Federation (WHF)
Past President, European Society of Cardiology (ESC)
University of Lisbon, Portugal
Introduction
For more than 26 years now, starting after the publication of the Consensus on Severe
Heart Disease in 1993,
2
the Brazilian Society of Cardiology (SBC) has been regularly issuing guidelines on
most diverse topics, guiding the practice of cardiology in Brazil. In 1999, the Brazilian
Federal Council of Medicine (CFM)
3
partnered with the Brazilian Medical Association (AMB) and, aiming to support medical
decision making and optimize patient care, started a process along with specialty
societies for the development of Medical Guidelines based on current scientific evidence.
Thus, the commitment of SBC precedes the initiative by AMB and fulfills one of the
society’s objectives, described in the society’s bylaws.
Resolution 1.642/2002,
4
passed by the CFM to preserve the autonomy of the physician, defined that, in their
relationship with physicians and beneficiaries, health insurance and group medical
companies, medical cooperatives, self-management companies, and other companies offering
direct care or care mediated by medical-hospital services should only adopt medical
guidelines or protocols prepared by Brazilian specialty societies along with the AMB.
Within this context,
5
the CFM initiated discussions in 2018 to update the regulations of telemedicine.
Telemedicine can be defined as the application of information and communication technologies
to health care with the goal of offering, in a broad concept, health-related services
ranging from primary care to robotic surgery and education, expanding coverage to
remote areas in a country with continental dimensions.
The Pan American Health Organization (PAHO) and the WHO define telemedicine as “The
delivery of health care services, where distance is a critical factor, by all health
care professionals using information and communication technologies for the exchange
of valid information for diagnosis, treatment, and prevention of disease and injuries,
research and evaluation, and for the continuing education of health care providers,
all in the interests of advancing the health of individuals and their communities.”
The PAHO estimates that one third of the population in the Americas has no access
to health care and that 800,000 additional health care professionals would be needed
to meet the needs in the region.
6
If applied in its broad context, telemedicine could allow access and reduce inequality
for this population by providing supposedly cost-effective quality services, especially
considering the increased prevalence and mortality from chronic noncommunicable diseases
(NCDs) in low- and middle-income countries like Brazil. Added to this context is the
aging and increasing disease rate of the Brazilian population, which makes telemedicine
an ideal tool to face the contemporary challenges of universal health care systems.
7
Beyond the vast possibilities and applications of telemedicine, rigorous evaluations
of telemedicine projects must be undertaken, not only because all health care systems
face financial sustainability challenges beyond investments in health care interventions,
but also because of the limited clinical evidence available, especially in the current
order of value-based medicine. This topic of utmost importance has been the subject
of several publications by the WHO. Examples of that include the Digital Health Atlas,
8
a global virtual platform to support governments in monitoring and coordinating digital
health activities; “BeHe@lthy, BeMobile” (BHBM),
9
for the prevention and control of NCDs; and mHealth Assessment and Planning for Scale
(MAPS), a manual for digital health monitoring and evaluation
10
to enhance digital health research and implementation; among others. These documents
culminated in the publication by the WHO of the first guideline on digital health
interventions on April 17, 2019.
11
In addition to updating the guideline on telemedicine applicable to cardiology published
in 2015, the main objective of the present guideline is to answer the following questions:
Is there legal and ethical support for the application of telemedicine in Brazil?
Are there technical conditions for the application of telemedicine in the country?
What is the priority of incorporating telemedicine into the health care system? For
which modalities is there good quality scientific evidence to support this practice?
For modalities supported by solid evidence, does cost effectiveness justify this application?
What would be the budgetary impact? Is the Brazilian health care system prepared to
provide comprehensive care?
This guideline, which is in line with the WHO guidelines,
11
advocates that the implementation of telemedicine should be a planned process that
provides feasibility of the network coverage in remote locations, elaboration of the
legal and judicial bases for its implementation, budgetary impact and cost-effectiveness
assessment of each stage of the project, and development of clinical continuum indicators
of the applicability for the safety of the beneficiaries. Telemedicine can be a potential
tool in improving health care services but is not exempt from risks and challenges
related to its implementation and from the evaluation of the real impact of its benefits.
In the final chapter, the authors present a summary of recommendations based on current
evidence, in an attempt to guide the discussions that will certainly permeate the
democratization of comprehensive health care services, especially the actions involving
telemedicine as a tool to expand the universality and integrality of the Brazilian
Unified Health System (SUS), recommendations that also extend to supplementary health
care.
Brazil, June 2019.
Dr. Marcelo Antônio Cartaxo Queiroga, FESC, TEC-SBC
President-elect of the Brazilian Society of Cardiology (Sociedade Brasileira de Cardiologia
- SBC)
Director of the Department of Interventional Cardiology, Alberto Urquiza Wanderley
Hospital, João Pessoa, PB, Brazil Member of the Paraíba State Academy of Medicine
Dr. Gláucia Maria Moraes de Oliveira, FACC, FESC, TEC-SBC
Associate Professor of Cardiology at the Federal University of Rio de Janeiro (Universidade
Federal do Rio de Janeiro - UFRJ)
Coordinator of the Postgraduate Cardiology Program at UFRJ, Rio de Janeiro, RJ, Brazil
President of the Federation of the Cardiology Societies of the Portuguese-Speaking
Countries (2015-2016)
1. Fundamentals of Telemedicine: Concepts, Bioethical Aspects, Legislation and Regulation,
Applicability in Brazil, and Artificial Intelligence
1.1. Fundamentals of Telemedicine
In May 2005, Ministers of Health from 192 countries members of the World Health Organization
(WHO) approved the Resolution on eHealth,
12
which recognized for the first time the importance of information and communication
technologies (ICTs) applied to health - digital health or eHealth - “reinforcing the
fundamental human rights by increasing and improving equity, solidarity, quality of
life, and quality of care.”
The Brazilian Ministry of Health defines the following areas of telehealth application:
13
Innovation in digital health and telehealth
Innovation in digital health is transversal to telehealth initiatives and seeks to
explore via ICT new ideas to solve chronic problems with difficult solutions by usual
methods. It must start with the population’s health care needs.
Teleconsulting
Registered consultation between health care workers, professionals, and managers using
two-way telecommunication instruments in order to answer questions about clinical
procedures, health care actions, and suggestions related to the work process in health
care. Teleconsulting can occur in real time or by offline messaging.
Telediagnosis
Autonomous service using ICT to deliver diagnostic support services (e.g., remote
evaluation of diagnostic tests) to facilitate access to specialized services. The
use of telediagnosis seeks to reduce the time to diagnosis by enabling treatment for
predictable complications through early diagnosis.
Telemonitoring
Remote monitoring of patients’ health and/or disease parameters through ICT. Monitoring
may include clinical data collection, transmission, processing, and management by
a health care professional using an electronic system.
Teleregulation
Set of actions in regulatory systems for evaluation of adequate responses to existing
demands, promoting equity and access to services, and enabling health care access.
Teleregulation also includes the evaluation and planning of actions to provide regulatory
operational intelligence to management teams. The objective of teleregulation is to
potentiate primary health care services, thus enabling the qualification and reduction
of wait for specialized care.
Tele-education
Availability of interactive educational materials on health-related topics delivered
remotely through ICT and focused on professional education across activity areas.
1.2. Types of Intervention in Telehealth
Synchronous video conference: modality of remote interaction via live conference between
primary care and medical specialty services.
Asynchronous video conference (“store and forward”): use of a storage system to forward
diagnostic images, vital signs, and/or video clips along with patients’ data for later
review by a specialist. Provides diagnostic and treatment support for the primary
care system.
Remote monitoring: use of equipment to remotely collect and forward patients’ data
to a hospital or monitoring center for interpretation. These (wearable) devices monitor
remotely a variety of indicators ranging from specific vital signs (heart rate, blood
pressure [BP], and blood glucose) to other indicators.
Mobile health (mHealth): defined as a medical and public health care practice supported
by mobile devices like cell phones, monitoring devices, personal digital assistants
(PDAs), and other wireless devices.
14
The goals of telemedicine include:
Remote assistance: teleconsultation, telediagnosis or diagnostic telemonitoring, remote
patient monitoring and/or treatment;
Administrative management of patient care: request of diagnostic tests, medical prescriptions,
and actions related to service reimbursement;
Remote qualification of human resources to facilitate continuing education programs;
Network collaborative clinical research: use of ICT to share and disseminate best
practices and generate knowledge.
1.3. Safe Bases for Data Transmission
Information safety is fundamental for data transmission, and two immediate effects
must be considered: a) understanding of the critical value of data storage and use,
and b) possible implications for individuals and organizations of violating safety
and compliance standards.
The European General Data Protection Regulation (GDPR) and the Brazilian General Data
Protection Act (Lei Geral de Proteção de Dados, LGPD) impose heavy fines and sanctions
for improper access to information under their custody.
The following sections list the main requirements for establishing appropriate safety
policies.
15
1.4. Data Protection and Confidentiality
For proper information protection, the safety of the systems must be ensured, reducing
vulnerabilities and preventing improper access and breach of confidentiality. Authorizations
and hierarchical levels for access to information must be clearly determined.
16
The policy related to information access and confidentiality must be reported in a
document signed by the users defining the a) scope of data that can be accessed and
b) legal implications and sanctions eventually applied to users in case of violation
of the agreed rules.
Misuse of technological installations is directly related to the safety of the environments
under the responsibility of ICT teams. Strict policies must be adopted in terms of
access to physical facilities, data networks, operating systems, and databases and
their applications. A valuable framework to provide an understanding of the control
of these environments can be found in the document “Access Control Example Policy”
(Health and Social Care Information Centre, 2017).
16
The recommended standard for data transmission in Brazil follows the set of rules
determined by the Health Insurance Portability and Accountability Act (HIPAA).17 This
set of norms has proven robust enough to ensure the safety of the transferred data
and is recommended as the benchmark for data transfer practices. The CFM Resolution
2.227/2018, now revoked, set the standard that would meet the desirable requirements:
“Use of a proprietary or an open-source electronic/digital information registration
system that captures, stores, presents, transfers, or prints digitally identifiable
health information and is fully compliant with the requirements of Safety Assurance
Level 2 (Nível de Garantia de Segurança 2, NGS2) and the ICP-Brazil standard.”
According to these standards, stored data (“at rest”; “in transit”) must be encrypted
for transfer. One of the essential practices for data security is to maintain the
tools required to encrypt and decrypt information in environments other than the original
storage locations.
18-20
In addition to ensuring information security, HIPAA rules offer extensive documentation
for data encryption and transfer, facilitating the work of development teams. Of note,
national public data cannot be stored in cloud systems hosted outside the country.
21-22
1.5. Bioethical Aspects
Initiatives to provide remote health care through telemedicine date back to the 19th
century. Cardiology was a pioneer in this initiative, with the description by Einthoven
in 1906 of a transtelephonic electrocardiographic transmission from the academic hospital
to the physiology laboratory at Leiden University, a few miles away.
23
The big boost in the development of telemetry was by the North American Space Agency
(NASA) in astronaut monitoring.
24,25
However, the incorporation of telemedicine, as currently conceived, is contemporaneous
24-29
and linked to the traditional notion that the preservation of the social value of
medicine depends on content flow. Any modality of telecommunication holds both constructive
and destructive potentials that trigger contradictions in terms of values and rules
of moral code related to bedside medical practice. Ambivalence is welcome in medicine,
which according to Osler (1849-1919), is the science of uncertainty and the art of
probability.
28
Telemedicine is not immune to the pendular movements of the variety of methods addressing
health needs.
Bedside practice faces dilemmas inherent to the diversity of the human condition.
30
Physicians and patients face external and/or internal challenges without a single
and simple solution. Any option to be considered must be judiciously expressed, clarified,
and adjusted to be validated for the conceptual and individual context of the clinical
circumstance.
Applied technology has attributed a sense of real progress to medicine.
31
The contemporary emphasis on ICTs in health care must be critically observed by society.
Bioethics has the required competence to evaluate the effects of telemedicine on the
integration of health sciences, health care professionals, patients/relatives, health
institutions, and health care system.
The benefit of telemedicine should be considered more as a non-presential complementation
of usual care rather than a replacement for face-to-face care. Telemedicine should
be practiced with security and for a period relevant to the clinical circumstance
(expiration dates proportional to the legitimate interests involved).
32,33
An additional ethical aspect is that certain unavoidable perspectives of abuse of
a technique should not adversely affect the beneficial use of the technique. Therefore,
any ethical and legal considerations regarding the still young telemedicine, especially
for application in a continental, multiethnic, and multicultural country like Brazil,
cannot fail to recognize that it is difficult for a health care professional to define
comprehensively and in depth his or her set of responsibilities, considering that
the scope of telemedicine demands an A-to-Z range of intertwining requirements, decisions,
and provisions regarding:
involvement with fundamentals of current ethics, prudence, and zeal regarding complex
issues like elderly care, comfort of vulnerable individuals, decrease in hospitalizations,
and prompt guidance;
impartial judgment about covering the patient’s real needs and constraint of secondary
gains and conflicts of interest, including the potential for political (mis)use and
power;
sense of beneficence;
avoidance of maleficence;
commitment to the biological safety of the patient;
respect for equity;
definition about the complementary function of the “presential” and its substitute;
awareness about the consequences of the “non-presential” on clinical reasoning;
clarity about the range of use variations;
training on roles, responsibilities, and skills in equipment management with continuous
improvement;
development of a friendly connection to the patients’ records;
respect for the patient’s right to autonomy expressed through free, informed, renewable,
and revocable consent;
imperative appreciation of human values;
critical appreciation of cost effectiveness;
appreciation of the value of face-to-face relationships immediately or long before
the online connection;
creation of a mood of confidence despite the distance;
conceptual and event-driven alerts about non-presential limitations;
individual assessment of the level of competence for the required care at the moment;
assessment of the completeness of the required information;
concern with the continuity of the care provided;
promotion of adherence to the recommended management;
respect for professional confidentiality;
“passport adjustments” related to the state geographical limitation of the physician’s
registration in the medical council;
continued research for reliable evidence of advantages and disadvantages;
interface with consumerism in health care, including due and undue expectations about
the possibility of immediate care;
valuing the contribution of bioethics to the harmonization between classic, innovative,
and novelty.
Therefore, in light of the existing ethical-normative bases of the current legislation
and the bioethical principles that guide physician-patient relationships, we can establish
the following guidelines for the use of telemedicine in cardiology:
Cardiologists should use caution, and prior to using telemedicine applied to cardiology
(or simply telecardiology), they should maintain a fruitful relationship with their
patients based on the Code of Medical Ethics.
The free and informed consent form is the document that obtains authorization from
the patient for the use of telecardiology when the alternative of direct teleconsultation
is considered.
Procedures for the remote monitoring of vital signs should be previously consented
by the patient, with proper guidance and training regarding its use.
Medical companies providing telecardiology services must be registered with the Regional
Medical Council (Conselho Regional de Medicina, CRM) and have a cardiologist as a
technical manager, who will be in charge of overseeing the procedures performed, especially
regarding the technological tools available to professionals.
Respect for the patients’ autonomy of will and protection of privacy regarding health
data are the basis of telemedicine applied to cardiology.
1.6. Legislation and Regulation in Brazil
The Brazilian Internet Civil Framework (Federal Law No. 12.965, dated April 23, 2014)
34
and the General Data Protection Law (LGPD; Federal Law No. 13.709, dated August 14
2018)
35
are the main normative instruments with direct impact on telemedicine in Brazil, even
though they are not specific for this purpose. The main authorities regulating telemedicine
in Brazil are the Ministry of Health, the National Sanitary Surveillance Agency (Anvisa),
the National Supplementary Health Agency (ANS), and the CFM.
1.6.1. The Brazilian Internet Civil Framework
The Brazilian Internet Civil Framework (Decree 8.771, dated May 11, 2016)
34
establishes the principles, guarantees, rights, and duties of users of the World Wide
Web in Brazil.
The Brazilian Internet Civil Framework recognized legal relations in the virtual world
and their effects on Brazilian order. In addition to establishing the neutrality of
the web, it also excelled in safeguarding freedom of expression and privacy protection
but failed to address important aspects related to personal data, leading to the development
of the LGPD.34
1.6.2. General Law Data Protection Law
Federal Law No. 13.709,
35
dated August 14, 2018 (LGPD) deals with the processing of personal data, including
digital information, by an individual or entity governed by public or private law,
with the purpose of protecting the fundamental rights of freedom and privacy and the
free development of the personality of the natural individual.
An important contribution of the LGPD
35
is the clear definition of the concept of data:
Personal data - information related to an identified or identifiable natural individual;
Anonymized data - data related to a holder that cannot be identified by reasonable
technical means available at the time of processing;
Sensitive personal data - racial or ethnic origin; religious belief; political opinion;
union affiliation or membership in religious, philosophical, or political organizations;
health- or sex-related data; and genetic or biometric data, when linked to a natural
individual.
According to the legislation, access to the Internet is essential to the exercise
of citizenship, and inviolability of intimacy, privacy, and communications established
through the Internet must be ensured to users.
Safety and confidentiality measures and procedures must be clearly informed by the
service provider and should meet the standards set by regulations, respecting the
right of confidentiality related to business privacy.
Regarding telemedicine, the need to deal with a large amount of sensitive data (patient
registration, health complaints, prior and current disease history, test requests
and results, diagnostic hypotheses, therapeutic plan, clinical follow-up, and opinions,
among others) makes LGPD an object of significant interest.
In 2007, the Brazilian Ministry of Health established the National Telehealth Program
to improve the quality of primary health care in the Unified Health System (SUS) and
support the Family Health Strategy program. Ordinance 2.546, dated October 2011, expanded,
redefined, and renamed the program to Brazilian National Telehealth Network Program,
which governs the services of synchronous or asynchronous teleconsultation, telediagnosis,
second formative opinion, and health tele-education.
36
1.6.3. Regulation of Telemedicine by the Federal Council of Medicine
According to CFM Resolution 1.643/2002,
37
telemedicine is the practice of medicine through the use of interactive audiovisual
and data communication methodologies, with the objective of health care, education,
and research. Additionally, the following relevant aspects should be highlighted:
The services provided must have appropriate technological infrastructure and should
comply with the CFM technical standards related to data storage, handling, transfer,
confidentiality, and privacy, and must ensure professional secrecy.
The professional responsibility for the care lies with the patient’s attending physician.
Others involved in the process will be jointly liable to the extent to which they
contribute to the eventual damage.
Entities providing telemedicine services must be registered in the Entities Register
of the Regional Council of Medicine of the state of their location along with a physician
regularly registered in the Council assigned as a technical manager and a list of
all physicians participating as staff members.
Since then, technological innovations and the democratization of Internet access have
allowed several innovations that still lack proper regulation, such as:
new means of physician-patient relationship;
emergence of data and service agents and providers;
discussion of a new format for the free and informed consent form under strict safety
rules to guarantee information confidentiality and integrity.
This scenario prompted a need to update the regulation of telemedicine practice in
Brazil. Based on that, the CFM issued Resolution 2.227/2018, which was later repealed.
However, an update of the Resolution is urgently needed to provide legal security
within the perspective of telemedicine emerging as a vector of health transformation.
38
In this guideline, we adopt the denomination of the services offered within the scope
of telemedicine, according to the Ministry of Health Ordinance 2.546, dated October
2011, and current CFM regulation.
1.7. Applicability in Brazil
In a country with continental proportions like Brazil, telemedicine represents a perspective
to ensure the implementation of public policies conceived when the SUS was established,
which have not been entirely fulfilled due to existing unassisted or remote areas
lacking health care professionals, among other reasons. Thus, infrastructure conditions
must be established to deliver available resources using health-related ICTs to these
areas. To understand the applicability of telemedicine in Brazil, it is important
to discuss concepts related to remote areas and to know the country’s medical demography.
1.7.1. Concept of Urban and Rural Territories and Remote Area
The definition of territory goes beyond that of physical space since it generally
has a strong relationship with the sociocultural context of the area. The division
between urban and rural spaces is not abrupt; both have flexible boundaries and similar
characteristics.
39
Territorial occupation is evidently unequal in many regions, as it is also the access
to goods and services offered in different forms of human settlements. In general,
modes of transport and accessibility to urban and rural areas differ from one location
to another, thus the importance of defining a classification for urban and rural concepts.
40
According to the Organization for Economic Cooperation and Development (OECD), spaces
are classified according to the population density, the proportion of the population
living in large centers, and accessibility, defined as the commuting time between
urban centers and rural areas. A rural area is classified as remote by the OECD when
50% of the local population requires at least 45 to 60 minutes of travel in motor
vehicle to reach a center with a population of at least 50,000 inhabitants.
41
In Brazil, the classification of occupied rural or urban spaces was established in
1938 by Decree No. 311/1938. The 2014 Territorial Base Manual, by the Brazilian Institute
of Geography and Statistics (IBGE),
42
considers the access by national road or waterway network from rural areas to urban
centers to classify rural areas according to their degree of proximity and access
to large urban centers, creating a sense of isolation. The 2014 Transportation Logistics
Map classified municipalities as adjacent or remote if the travel time from the municipal
headquarters to a center of influence was longer or shorter, respectively, than the
national average.
Table 1.1 shows the distribution of municipalities across the national territory based
on the classification of isolation by IBGE.
43
Table 1.1
Classification of isolation of Brazilian municipalities according to region and population
43
Classification of isolation (IBGE)
Brazil
North
Northeast
Southeast
South
Midwest
Adjacent
Number of municipalities
5,126
277
1,683
1,637
1,180
349
% of municipalities in relation to the large region
92.11
61.69
93.81
98.14
99.33
74.89
Total population2010 Census)
183,820,219
12,610,201
51,780,322
79,982,805
27,099,304
12,347,587
% of the population
96.37
79.49
97.56
99.53
98.95
87.83
Remote
Number of municipalities
439
172
111
31
8
117
% of municipalities in relation to the large region
7.89
38.31
6.19
1.86
0.67
25.11
Total population (2010 Census)
6,927,512
3,254,253
1,293,560
381,605
287,587
1,710,507
% of the population
3.63
20.51
2.44
0.47
1.05
12.17
Source: IBGE. Classification and Characterization of Rural and Urban Spaces in Brazil.
43
More than 65% of the municipalities considered to be remote are located in the North
and Midwest regions of the country. These two regions concentrate 5 million inhabitants
or 72% of the country’s residents living in remote municipalities (almost 7 million
individuals live in areas considered remote by the IBGE). Also in the North and Midwest
regions, the population in remote municipalities represents 20% and 12% of the total
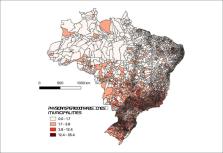
population, respectively. Figure 1.1 shows the proportion of urban population in Brazilian
municipalities.
Figure 1.1
Proportion of urban population in Brazilian municipalities, 2010 census.
44
Source: IBGE – 2010 census.
44
1.7.2. Medical Demography
The ratio of physicians per inhabitant in Brazil (2.1 physicians per thousand inhabitants)
is significantly lower than the average ratio in OECD countries (3.4 physicians per
thousand inhabitants). In addition to the absolute shortage of professionals, the
country also has relative shortages due to large regional inequalities in the distribution
of the existing medical workforce. Recent studies point out to a large concentration
of medical professionals in the South and Southeast, with the proportion of specialists
following this trend.
45
Table 1.2 shows the distribution of physicians by country region, divided according
to specialization as generalists, with some type of specialty (specialists), ratio
per thousand inhabitants, and distribution of cardiologists by region and per inhabitant.
Table 1.2
Distribution of physicians by region of the country, disaggregated by specialization
and region, grouped as generalists or specialists
Region
Physicians
Generalists
Specialists
Population
Cardiology
Cardiology/1,000 inhabs.
Physician/1,000 inhabs.
North
20,884
10,128
10,766
17,936,201
441
0.025
1.16
Northeast
80,623
34,461
46,162
57,254,159
2,534
0.044
1.41
Midwest
37,536
12,828
24,708
15,875,907
1,464
0.092
2.36
Southeast
244,304
91,124
153,180
86,949,714
8,383
0.096
2.81
South
68,430
20,948
47,482
29,644,948
2,694
0.091
2.31
Source: Scheffer M, Cassenote A, Guilloux AGA, Mioto BA, Mainardi GM. Medical Demography
in Brazil 2018. São Paulo: FMUSP, CFM, Cremesp; 2018.
45
*Population estimated by IBGE in 2017.
In the North and Northeast regions, some Federation units have a physician/inhabitant
ratio below 1.00, like Pará and Maranhão, where the ratios are 0.97 and 0.87, respectively.
The most recent Brazilian Medical Demographic Report (2018) also pointed to a significant
inequality in the distribution of physicians between predominantly urban and rural
municipalities, with a high concentration of the medical workforce in large urban
centers.
45
Data from the National Register of Health Establishments (Cadastro Nacional de Estabelecimentos
de Saúde, CNES), provided by the Ministry of Health,
46
show the same trend of concentration of medical professionals in the South and Southeast
regions in February 2019, as seen in Figure 1.2.
46
Figure 1.2
Distribution of physicians per thousand inhabitants in Brazilian municipalities –
CNES, February 2019.
46
Source: National Register of Health Facilities (CNES/DATASUS/MS).
46
1.7.3. eHealth Strategy
The International Telecommunication Union (ITU),
47
an agency of the United Nations (UN), has been working in collaboration with the WHO
to create a global environment for eHealth strategy implementation, especially in
telemedicine.
47,48
The eHealth strategy is particularly important in the control of chronic noncommunicable
diseases like hypertension, diabetes, heart diseases, and age-related diseases. The
implementation of eHealth and telemedicine has progressed substantially in recent
years,
49
but a recent systematic review on the cost effectiveness of eHealth implementation
found shortage of studies and could not assess the impact of the strategy on health
systems or social aspects, although most studies showed the strategy to be efficacious
and cost effective.
49
1.7.4. Telecommunications and Data Infrastructure
Up to 95% of the world’s population is estimated to have access to mobile telephony;
in Brazil, this coverage may exceed 98%. Access to mobile phone services has progressed
remarkably in Brazil, and the use of mobile phone equipment per inhabitant has increased
from 2009 to 2019,
50,51
followed by a downward trend since then (Figure 1.3). Figure 1.4 shows the distribution
of cell phones per 100 inhabitants and the ratio between cardiologists and cell phones
per 1,000 inhabitants in Brazil in 2018.
Figure 1.3
Density of access to mobile phones in Brazil and regions, March 2009 to 2019.
50
Source: ANATEL.
50
Figure 1.4
Distribution of cell phones and cardiologists, Brazil. a) Ratio cardiologists/1,000
inhabitants (2017), b) Density of cell phone density/100 inhabitants (2019).
51
Source: Scheffer M, Cassenote A, Guilloux AGA, Mioto BA, Mainardi GM. Medical Demographics
in Brazil 2018. São Paulo: FMUSP, CFM, Cremesp; 2018.
45
In terms of optical fiber coverage, the concentration is also greater in the Brazilian
South and Southeast regions. Figure 1.5 shows the distribution of optical fiber backhaul
in Brazilian municipalities. Backhaul is the portion of a hierarchical network (like
cellular mobile communication networks) that is responsible for connecting the main
network and the subnets. As shown in the map in figure 1.5, the concentration of optical
fiber networks is lower in municipalities of the North region, which also concentrates
the largest proportion of isolated municipalities.
Figure 1.5
Municipalities with optical fiber backhaul and other technologies, February 2019.
Source: ANATEL.
50
Data from figures 1.4 and 1.5 show a trend of concentration of cardiologists in areas
with a higher concentration of enabled mobile devices. The correlation coefficient
of this relationship is 0.67, which indicates that the availability of cardiologists
correlates highly with access to mobile phones. These data indicate a greater challenge
to the implementation of telemedicine in remote areas, considering that the shortage
of physicians follows the same distribution of the deficient telecommunications infrastructure
in Brazil. A detailed analysis of the costs and benefits of this expansion should
direct incentives to this area.
1.8. Artificial Intelligence
Artificial intelligence (AI) is a complex framework of sophisticated mathematical-computational
models that allows the construction of algorithms to emulate various human tasks.
AI encompasses an increasing number of subareas translating into different combined
or complementary methodologies and approaches. Some examples include artificial neural
networks (particularly deep learning models and convolutional networks), support vector
machines, evolutionary algorithms, and natural language processing. The elaboration
of analytical algorithms derived from large databases allows for interactive interpretation
and apprehension, recognition of hidden patterns of combined information not obtained
with traditional statistical methods, and assistance in more accurate decision making.
The availability of this huge amount of data and new analytical techniques - big data
analytics - opens up new scientific possibilities and AI applications, such as machine
learning and data mining, which are already widely applied in telecardiology to diagnose
combinations of multiple modalities of images, biobanks, electronic cohorts, on-site
and distance clinical monitoring sensors, electronic health records, genomes and other
molecular techniques, among others.
52-54
The implementation of these applications in clinical cardiology has grown exponentially
55
and has prognostic features, like the use of an algorithm derived from magnetic resonance
based on three-dimensional patterns of right ventricular systolic function to assess
with high accuracy the outcomes in pulmonary arterial hypertension,
56
identification of phenotypic patterns in heart failure with preserved ejection fraction
and unfavorable prognosis confirmed by heterogeneous patterns of ventricular repolarization
on electrocardiogram,
57
prediction of cardiovascular risk in large cohorts,
58
and prediction of urgent revascularization in emergency patients with chest pain,
59
among others. However, AI studies are generally based on observational data from administrative
databases or clinical records, which potentially have different types of biases and
confounding factors.
54
AI applications in telemedicine are promising but still very limited.
60
In the area of telediagnosis, efforts for automated classification and diagnosis in
electrocardiography and cardiovascular imaging
61
are promising but still incipient. As for cardiovascular interventions, a recent review
62
found 8 studies incorporating machine learning in a real-life research setting, of
which only three were evaluated in a randomized controlled trial. Of the 8 interventions,
6 (75%) showed statistical significance (at a p level of 0.05) in health outcomes.
Some of these interventions are directly related to telecardiology and assessed interventions
for weight loss, stress control, smoking cessation, and personalized nutrition based
on glycemic response. Most studies had small sample sizes and short duration, reflecting
a need for investments and further studies exploring the potentialities in the area.
In a recent review, Topol
63
highlighted the presuppositions that will guide the future of AI in medicine: the
patient must be considered the center for the implementation of any new technology,
the incorporation of these new technologies for diagnosis and treatment should occur
after robust validation of their clinical effectiveness, the use of digital tools
and decision algorithms by patients should be an option for those who feel empowered
to do so, and interdisciplinary training must involve health care professionals, engineers,
computer scientists, and bioinformaticians. These minimum conditions presuppose the
steps to incorporate AI into clinical practice and minimize implementation challenges.
However, many aspects of health care practice will continue to depend on other dimensions,
such as political, economic, and cultural ones, and on the ability of the health care
professionals to interact with patients and community so that AI can truly benefit
the patients, given that the issue of unequal access to health care is still critical
in Brazil and will require large investments and reorganization of the health care
system.
54
Thus, potential strategies for incorporation and planning of implementation and adoption
must be aligned with the possibility and challenges of offering cardiology care centered
on the patient and the final value aggregated to the line of cardiology care. There
is a need to identify the best technology to incorporate and define in which part
of the medical work process such technology can add value to both the process and
the patient’s health. Additionally, it is necessary to plan the incorporation and
design the journey of digital transformation in cardiology to ensure a high technological
level.
The incorporation of these technologies into clinical practice must, at first, involve
rigorous evaluation of their performance and their ultimate value for the patient.
This evaluation should respect and follow the current evaluation process of incorporation
of new health technologies by the Ministry of Health, considering all aspects, norms,
and regulations. The incorporation should also be based on scientific evidence on
the generation of ultimate value to the patient’s health from the perspective of an
individual exposed to technology.
It should be made clear that AI, once incorporated, works by increasing the professionals’
capabilities and never by replacing them, and that all civil and criminal responsibilities,
as well as all responsibilities related to the patient and his or her health problem,
remain with the attending physician.
64
Training should be multiprofessional, interdisciplinary, and focused on building services
dedicated to generating the ultimate value for the patient. The medical curriculum
in the cardiology area should include contents related to technical knowledge, competence
development, and use of AI techniques, while cardiology services should structure
a continuous program for professional qualification and human resources training in
managing incorporation, training, and adoption of new digital health technologies.
At present, there is no specific regulation on the use of AI in health care, although
countries like Canada, United Kingdom, and the United States have begun the first
phases of planning and implementing AI regulation in this area. Also, the European
Union has published a document on the ethical aspects of AI in health care.
63
The fast-paced digital transformation has led to reflections on how to balance the
adoption of technology and emerging digital systems with ethical, moral, emotional,
and social values, particularly values related to patients’ safety.
2. Uses and Application of Telemedicine in Cardiology
2.1 Telemedicine in Brazil
With the development of the Information Society in the late 20th century as a result
of globalization and widespread use of ICT, the emergence of organizational, social,
political, and economic innovations of the society became pressing issues, requiring
new ways to learn, teach, and work. The world began to worry about the principles
of equal opportunity, participation, and integration so that everyone could access
and benefit from the applications of the Information Society. In health care, telemedicine
has made substantial progress worldwide, as it is classically viewed as a set of actions
with great potential to improve access to health care services and to care quality
and effectiveness at a lower cost.
65
As a mark of the new millennium, we highlight the aging of the population, the increase
in chronic noncommunicable diseases, and the consequent need to provide health care
services for a longer time, which increases health-related costs. Therefore, it is
essential to incorporate innovative, efficient, and effective solutions like telemedicine
and biotechnology to promote universality and comprehensiveness in health care.
Several actions in telemedicine are currently present in all continents and must be
planned according to local needs in order to be successful. According to Bashshur
et al.,
66
the success of these actions depends on three pillars: access, quality, and cost.
66
In developed countries, telemedicine is an alternative to traditional methods and
is already present as an option to supplementary health or to address gaps in the
health care system, but always aiming at integral care. In developing countries, access
is the main pillar, since telemedicine can be the only option in regions where traditional
specialized care is not available.
In Brazil, the systematic development of telemedicine and telehealth in the public
health system started in 2006, with investments from the Ministry of Health, State
Health Secretariats, and Municipal Health Secretariats. The main objective was to
support primary care, particularly the Family Health Strategy in remote municipalities,
through teleconsulting, telediagnosis, and tele-education. If applied on a large scale,
these strategies could decrease the referral of patients to large centers and consequently
improve the population’s access to specialized care and reduce health care costs.
67
Therefore, telemedicine in the Brazilian public system has been anchored from the
outset in the basic principles of universality, equity, and integrality of the SUS.
Based on the universality principle, health is everyone’s right, and it is up to the
state to ensure it. Equity targets the reduction of inequalities or increased investment
in areas where it’s most needed. Integrality considers the individual as a whole to
meet all his or her needs.
68
Telecardiology, one of the most developed domains in telemedicine, has multiple actions
in promoting health, disease prevention, diagnosis, treatment, and rehabilitation
with an impact on the quality of life. It can be considered an important ally of the
public, supplementary, or private health care system in promoting comprehensive and
high-quality health care.
2.2. In Primary Care
Primary Health Care (PHC) involves integrated and multidisciplinary care and is the
foundation to achieve universal health, according to the PAHO, which also advocates
for other health determinants like education, food, housing, financial protection,
clean water, and safe environments.
69
To achieve universal health, health care systems must be transformed, especially by
making PHC efficient, integrated, and organized, placing the patient at the center
of the system. The PAHO also estimates that about one third of the population in the
Americas has no access to health care and that 800,000 additional health care professionals
would be necessary to meet the needs in the region.
Telemedicine plays an important role in the qualification of the PHC, with clinical,
human, organizational, educational, administrative, technical, and social benefits.
70
The application of telemedicine to support PHC brings benefits to the population served,
including (i) improved access to specialized services, (ii) increased solvability
to the basic level, (iii) decreased number of patients referred to other municipalities
for specialized care, (iv) more qualified referrals and faster hospitalization decisions,
(v) better training of local health care professionals, with consequently better qualified
clinical care, (vi) reduced time to diagnosis, with decreased risk of complications,
(vii) diagnosis of diseases at earlier stages, (viii) cost and time savings for the
patient, (ix) improved quality of life, (x) fewer hospitalizations and visits to emergency
units, (xi) improved clinical care continuum, (xii) reduced risk factors and complications
from chronic diseases, and (xiii) savings for the health care system.
70-74
2.2.1. Applications in Health Promotion and Prevention
In cardiology, health promoting actions and primary and secondary prevention of cardiovascular
diseases translate into significant cost savings by reducing specialized consultations,
hospitalizations for clinical complications, and admissions to the emergency room.
71
Telemedicine can be useful in controlling risk factors for coronary artery disease;
improving blood pressure control;
75-78
reducing glycosylated hemoglobin levels in patients with diabetes mellitus;
79-81
improving lipid profile;
82,83
reducing weight, body mass index (BMI), or waist circumference in obese individuals;
77,84-86
and increasing the success of smoking cessation programs.
87
Several modalities of telemedicine can be applied in this regard, including cell phone
text/audio messaging systems, which have positive results in improving medication
adherence, changing eating habits, and increasing physical activity among patients
with hypertension, diabetes, and obesity, or after acute myocardial infarction (AMI).
86,88
24-hour monitoring systems on cell phones or monitoring center services have become
more frequent due to the development of specialized equipment with direct communication
with telemedicine systems such as stethoscopes, scales, thermometers, digital devices,
blood pressure equipment, remote monitoring of vital signs and implantable electronic
devices.
89,90
Simple watches have been transformed into monitoring systems equipped with technology
to report heart rate, stress level (skin humidity and temperature), optical BP, and
physical activity, among other parameters.
91-92
Several applications are available to guide the health care team and/or patients,
including applications focused on self-care.
89
2.2.2. Decision Support Systems
Clinical decision support systems (DSSs) provide knowledge and information from individual
patients to physicians and other health care professionals, or to the patients themselves,
to improve care quality and clinical outcomes. These systems are recommended by the
Community Preventive Services Task Force (CPSTF) in the prevention of cardiovascular
diseases despite being based on moderate- to poor-quality evidence.93 Applications
that have shown benefits include those improving screening for cardiovascular risk
factors, prescription of aspirin for primary prevention, and counseling on healthy
diet, physical activity, and smoking cessation.94 Due to that, these applications
may have wide applicability in PHC. Still, they have shown no evidence of reducing
emergency visits, hospitalizations, or cardiovascular events, although additional
studies are still needed. A study reported a lower mortality rate with an educational
strategy for health care professionals associated with DSS alerts compared with educational
strategy alone.
95
DSSs have been evaluated in pilot studies in Brazilian Basic Health Units (Unidades
Básicas de Saúde, UBS) in multifaceted interventions. This tool was proven feasible
in PHC in patients with hypertension and diabetes and for cardiovascular risk management,
with good satisfaction reported by the professionals and perceived ease of use,
96,97
although the number of questionnaire fields filled in by the professionals was low.
96
This may be related to the incipient implantation of electronic medical records in
UBSs, generating duplicate work, a factor that was inversely related to the successful
implementation of the DSS.
98
New initiatives are underway to assess the impact on clinical outcomes of the control
of patients with hypertension and diabetes in the Mucuri Valley, in Minas Gerais,
and the management of patients on warfarin, still the most widely used anticoagulant
in the SUS.
2.2.3. Teleconsulting
Teleconsulting systems have great applicability in PHC in terms of supporting health
care professionals in remote areas and qualifying and reducing the time to diagnosis
and treatment. As a tool with the important potential of increasing PHC solvability,
teleconsulting should be incorporated into the care process in health care units as
an integral part of the regulatory process of the municipality. This is an efficient
way to reduce the long wait for in-person consultations with a cardiologist. Although
teleconsultation has been extensively studied in our country,
99
only a few studies have evaluated in our population the impact of teleconsultation
on traditional health outcomes, like risk and mortality. A systematic review by Liddy
et al.
100
reported that teleconsulting systems were highly accepted by patients and health care
professionals and improved access to specialized care. A randomized trial in cardiology
assessed adverse events (including death, AMI, urgent or emergent cardiac catheterization
and/or angioplasty, and emergency room visits) in patients referred to teleconsultation
versus patients receiving a traditional referral. The group referred to teleconsultation
was more likely to have an appointment with the cardiologist and had fewer visits
to the emergency department.
101
2.2.4. Teleregulation
The demand for specialized care has been growing worldwide and surpassing the supply
of services while meeting limited access to specialists and long waiting times.
101
Interventions in telehealth, particularly involving teleconsultation for regulation,
have shown a great impact in reducing waiting time with the qualification of access
to specialists, avoiding unnecessary referrals, and at a lower cost. In Brazil, the
experience of teleconsultation for regulation (or teleregulation) has also reduced
the waiting time for specialized consultation, qualifying the access and optimizing
the use of resources, in addition to providing users with greater comfort and lower
impact on their routine.
102
-
104
Teleregulation also enables the classification of the risk of the demand for specialized
care. Protocols to guide health care have been established, and the final decision
regarding referral is defined along with the attending and teleconsulting physicians.
In addition to the mentioned gains from the user’s perspective, there is the process
of continuing education and professional qualification, increasing solvability in
primary care.
101-104
2.2.5. Telediagnosis
Tele-electrocardiography, the most common activity in telecardiology, is a simple
and low-cost technology for easy transfer of a small file using an Internet connection
with limited bandwidth. Thus, it can be easily incorporated into the PHC routine for
its great applicability and suitability for the infrastructure of PHC facilities in
remote and poor areas.
105,106
Tele-electrocardiography is widespread in both public and private settings in Brazil,
with several companies in the country delivering reports around the clock. In 2017,
the Ministry of Health launched the National Offer of Telediagnosis Project (Projeto
Oferta Nacional de Telediagnóstico, ONTD) to expand the diagnostic services of tests
conducted remotely in the most deprived areas of the country. Tele-electrocardiography
was the first modality of telediagnosis offered nationwide by a telehealth team specialized
in telecardiology. This project is an innovation in the management of a large-scale
national telemedicine project model, and the good results obtained have shown its
easy applicability and suitability for remote areas.
107
The application of AI to the large databases of diagnostic tests improves the ease
of the process of reporting and increases the accuracy of the tests.
61,108
In telecardiology, tele-echocardiography is a promising strategy for rationalization
of the access to complementary propaedeutics, early diagnosis, prioritization of referrals,
and organization of waiting lists. Initial evidence of tele-echocardiography application
derive from population-based screening studies, for example, a study conducted in
rural India, where more than 1,000 echocardiograms were performed in about 11 hours
and transferred to a cloud computing system for expert analysis using telemedicine.
109
The strategy proved feasible and showed good agreement between preliminary field diagnoses
and the reports by experts (k = 0.85), and an alarming 16% rate of major abnormalities
(including 32.9% of valvular defects). Even in high-income regions like the UK, evidence
has shown echocardiographic screening in primary care by nonspecialists to be an attractive
strategy, with clinically significant (moderate to severe) valvular disease observed
in 6.4% of the asymptomatic population aged ≥ 65 years and associated with socioeconomic
factors.
110
The strategy may be especially useful in Brazil, which has a presumably high burden
of undiagnosed cardiovascular disease and limitations in the provision of specialized
tests, including conventional echocardiography.
The tele-echocardiography strategy was first tested in Brazil in a program for screening
of rheumatic heart disease (the Rheumatic Valvular Disease Screening Program study;
Programa de RastreamentO da VAlvopatia Reumática - PROVAR). The study established,
at a research level, a routine for the acquisition of simplified imaging protocols
using portable and ultraportable devices by non-physicians (nurses and technologists),
which were uploaded to dedicated cloud computing systems for storage and remote expert
interpretation.
111,112
In addition to remote diagnosis, telemedicine was also used to train health care professionals
on basic echocardiographic principles through interactive online modules. After training,
health care professionals with different backgrounds were able to diagnose rheumatic
heart disease with accuracy.
111
The project reported a high prevalence of subclinical rheumatic heart disease (4.2%),
which is quite significant considering the current impact of the disease on public
health.
113
A similar strategy was subsequently applied in primary care. Professionals (physicians,
nurses, nurse technicians) from health care centers located in low-income regions
of metropolitan areas of Belo Horizonte and Montes Claros received online and in-person
training for the acquisition of a simplified echocardiographic protocol with ultraportable
devices and support by the project’s field team. Echocardiography was performed in
asymptomatic individuals of three age groups (17-20, 35-40, and 60-65 years) as a
screening method and in patients on a waiting list to undergo echocardiography or
who had this test requested by the family health care team. The results showed that
a) the strategy is feasible for the conditions found in Brazil and has the potential
to be expanded to other scenarios; b) the prevalence of echocardiographic abnormalities
in asymptomatic populations was high (above 20%) in general; c) among patients on
a waiting list for echocardiography, more than 50% had no significant abnormalities
on screening echocardiography; and d) the correlation with conventional echocardiography
was satisfactory.
112
A prediction score was developed from these findings, incorporating clinical data
and variables from the simplified echocardiogram.
114
Thus, tele-echocardiography may be a strategy for early diagnosis but is mainly an
instrument to prioritize and organize waiting lists in health care systems with limited
availability of tests and specialized consultations. However, the incorporation of
this model into Brazilian health policies depends on broad regulatory discussions
involving authorities, professional councils, and medical societies - especially regarding
simplified image acquisition by non-physicians.
The adoption of tele-echocardiography (Table 2.1) for the care of disadvantaged remote
communities has potential advantages, but this method still lacks robust scientific
validation with prospective controlled studies confirming its benefits to patients’
health and cost-effectiveness, among other challenges.
Table 2.1
Potential advantages and challenges for the adoption of tele-echocardiography in Brazil
Advantages
Challenges
Allow access to the method at remote locations
Lack of standardization of the components of tele-echocardiography
Optimization of clinical outcomes
Absence of scientific evidence confirming the impact on clinical outcomes
Reduction in the cost of transporting human resources to geographically distant areas
Absence of scientific evidence confirming cost-effectiveness; questions about reimbursement
and system costs
Reduction in the cost of transporting patients to tertiary centers
Uncertainty about adherence by local health care professionals
Reduction in the number of unnecessary echocardiograms
Veto of the Brazilian legislation to the work of non-medical operators (sonographers)
Lack of guidelines for training of operators
Medical-legal uncertainties
Legislative issues related to licensing, data storage, privacy, and confidentiality
2.2.6. Tele-Education
Remote educational activities in cardiology for health care professionals, offering
courses, lectures, and learning tools on clinical issues and care management, have
the added benefit of improving the quality of care. Educational activities for patients
should be encouraged for their health empowerment.
In remote municipalities with small populations, PHC is often the only level of local
health care, while their health care units receive patients with acute cardiovascular
diseases. Thus, telecardiology in PHC not only should qualify the care of chronic
diseases but should also support urgent care for ischemic diseases and arrhythmias.
Due to the myriad of applications of simple telemedicine tools, cardiology can be
considered one of the specialties most sensitive to the use of ICTs. The triad teleconsulting,
telediagnosis, and tele-education integrally applied to PHC and associated with tools
like DSS can make a difference in the quality of care for cardiovascular diseases,
especially hypertension, atrial fibrillation, heart failure, and AMI. Finally, teleregulation
can offer support to PHC, in terms of solvability at this level, improving access
to specialty care.
2.7. In Specialized Care
2.7.1. Heart Failure
Extensive literature has examined the use of telemedicine strategies to monitor patients
with heart failure with the objective of reducing hospitalizations associated with
increased morbidity, mortality, and costs and improving patients’ adherence and participation.
The interventions range from the application of traditional technologies like structured
telephone support, telemonitoring using innovative technologies with implantable or
wearable devices, DSS, and machine learning to predict complications.
115,116,117
The results are variable, but most demonstrate benefits. However, the use of these
strategies in clinical practice is still very limited due to regulatory, logistics,
and financial issues.
118
Telemonitoring may be invasive or noninvasive. Sensors are tools capable of detecting,
recording, and responding to specific information, e.g., patients’ vital signs, and
are increasingly embedded in smartphones and other mobile devices. Sensor logging
can generate large data sets that may be transmitted in real time to health care professionals.
119
Since multiprofessional intervention programs often have geographical, economic, and
bureaucratic barriers, telemonitoring can be a solution to promote care for patients
with heart failure.
115
Evidence about structured telephone support and noninvasive telemonitoring in patients
with heart failure has been summarized in a Cochrane systematic review of 41 studies.
Structured telephone support reduced all-cause mortality (RR 0.87, 95%CI 0.77-0.98;
n = 9,222) and heart failure-related hospitalizations (RR 0.85, 95%CI 0.77-0.93; n
= 7,030), both with moderate-quality evidence. Telemonitoring reduced all-cause mortality
(RR 0.80, 95%CI 0.68-0.94; n = 3,740) and heart failure-related hospitalizations (RR
0.71, 95%CI 0.60-0.83; n = 2,148), both with moderate-quality evidence.
119
Another meta-analysis
120
of 26 studies with 2,506 patients undergoing telemonitoring (including the transmission
of vital signs) observed a time-dependent effect. Short-term follow-up (up to 180
days) had better results for hard outcomes (like mortality), which were not maintained
during longer follow-up (1 year). Regardless of the follow-up duration, the strategy
was unable to reduce hospitalization. An increase in emergency visits in the telemonitoring
group raises the question of how an intervention that does not reduce hospitalization
could reduce the mortality rate. Perhaps the early detection of signs of decompensation
encourages a more frequent search for care and prompt treatment with diuretics and
vasodilators without requiring intensive therapy.
In the publication of one of its Clinical Protocols and Guidelines on Heart Failure,
121
the Ministry of Health analyzed several studies evaluating the benefits of telemonitoring
based on telephone follow-up, recommending for health care services to consider follow-up
using telephone support for patients with New York Heart Association (NYHA) functional
class III to IV heart failure after hospital discharge. The analysis showed an 18%
reduction in overall mortality with remote monitoring compared with usual care (RR
0.82, 95%CI 0.73-0.93). Telemonitoring also reduced by 23% (RR 0.77, 95%CI 0.68-0.88)
the risk of hospitalization due to heart failure. Of note, this recommendation should
be directed to patients with the potential of most benefits. There is no consensus
on the intensity and frequency of monitoring, but they should be performed focused
on clinical and educational guidance.
Evidence regarding the duration of hospital stay is more fragile and controversial.
Of seven studies on structured telephone support and nine on telemonitoring, only
one on each type of intervention reported significantly decreased hospital stay. Additionally,
nine of 11 studies on structured telephone support and five of 11 studies on telemonitoring
reported significant improvements in quality of life. Three of nine studies on structured
telephone support and one of six studies on telemonitoring reported reductions in
cost, while two studies on telemonitoring reported increased costs due to expenses
related to the intervention and increased medical management. Seven of the nine studies
that assessed heart failure knowledge and self-care noted significant improvements.
Despite acceptance by 76-97% of the participants, decreased adherence to the intervention
over time can be challenging, and was reported in the review at 55.1-65.8% with structured
telephone support and 75-98.5% with telemonitoring.
119
Machine learning techniques can be potentially valuable in remote monitoring of patients
at high risk of heart failure. Individual characteristics of these patients obtained
from the analysis of a large number of electronic medical records may help identify
those at greatest risk of unfavorable outcomes who could benefit from individualized
medical treatment.
122
The Seattle Heart Failure Model (SHFM), for example, is a machine learning framework
for the calculation of mortality risk in heart failure. The model considers various
clinical aspects obtained from electronic medical records to predict the prognosis
of the disease and incorporates the potential impact of therapies on patients’ outcomes.
This DSS was shown to be potentially useful in identifying patients with heart failure
at higher risk of unfavorable outcomes, but met implementation barriers, as it was
time consuming, expensive, required familiarity of the physician with computers, and
failed to take into account other clinical variables that were not included in the
collected data.
123
Evidence of the benefits of telemonitoring in heart failure has been recently confirmed
with the publication of the Telemedical Interventional Management in Heart Failure
II (TIM-HF2) study. This was a prospective, randomized, multicenter clinical trial
including 1,571 patients with NYHA class II or III heart failure hospitalized due
to heart failure within 12 months before the randomization and with an ejection fraction
of 45% or lower. The patients were then randomized to remote management or usual care
and followed up for up to 393 days.
124
The percentage of days lost due to unplanned cardiovascular hospitalizations and death
from all causes was 4.88% in the remote patient management group and 6.64% in the
usual care group (p = 0.04). Patients assigned to remote management lost an average
of 17.8 days/year compared with 24.2 days/year among patients assigned to usual care.
The hazard ratio (HR) for all-cause mortality was 0.70 (95%CI 0.50-0.96; p = 0.0280)
in favor of the teleconsultation group, but cardiovascular mortality was not significantly
different between both groups (HR 0.671, 95%CI 0.45-1.01; p = 0.0560).
124
New devices to monitor intracardiac pressure present the most compelling evidence
for the application of telemonitoring and use more advanced technologies. CardioMEMS
is a device that is percutaneously implanted in the pulmonary artery to transmit central
pressure values to a platform. When the pressure levels of the pulmonary artery rise
above a certain threshold, the physician receives an alert and a statement indicating
congestion or low output. Other devices for implantation in the right ventricle are
being used experimentally. The study CHAMPION (CardioMEMS Heart Sensor Allows Monitoring
of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients)
125
evaluated patients with NYHA functional class III heart failure across 64 centers
in the US. The patients were randomized by a centralized electronic system to a group
of management by CardioMEMS or to a control group.
In the monitoring group, the physicians used daily data from pulmonary artery pressure
measurements to guide treatment. After a follow-up of 15 months, the monitoring group
had a 37% reduction in hospitalizations related to heart failure compared with the
control group. The long-term follow-up of this study, in which the control group was
switched to receive pulmonary pressure monitoring, showed that these results remained
significant and clinically relevant over time.
126
2.7.2. In Hypertension
Telemonitoring strategies can also be applied for BP control, but they overlap the
self- monitoring approach. In the TASMINH4 trial, 1,182 patients were randomized (1:1:1)
to general titration of antihypertensive medication based on clinical readings by
a generalist (usual care group), self-monitoring (self-monitoring group), or self-monitoring
along with telemonitoring (telemonitoring group). The study found that the use of
BP self-monitoring to titrate antihypertensive therapy in poorly controlled hypertension
in primary care resulted in lower systolic BP without increasing the workload of the
clinical team. After 1 year, patients who had the medications adjusted based on self-monitoring
with or without telemonitoring had significantly lower systolic BP than those who
had the treatment adjusted based on BP measured during consultations. The BP values
in the telemonitoring group that received medication titration became lower faster
(at 6 months) than in those in the self-monitoring group, an effect that is likely
to further reduce cardiovascular events and improve long-term control.
127,128
Several studies also show that strategies for hypertension telemonitoring involving
a clinical pharmacist lead to a beneficial impact on BP control in the short and medium
term. Margolis et al.
129
evaluated the durability of the effect of such intervention after a follow-up of 54
months in a randomized cluster study among 16 primary care clinics and 450 patients
(228 receiving telemonitoring and 222 on usual care). Intensive intervention based
on telemonitoring maintained the effects for up to 24 months (12 months after the
end of the intervention), but lost efficacy in the long term.
129
A prospective observational cohort study monitored the BP levels before and after
an educational intervention and introduction to home BP monitoring (HBPM). In the
intervention group, 484 patients were instructed to track their BP levels using a
smartphone three to seven times a week. The mean BP levels improved from 42% to 67%
among patients on HBPM compared with 59% to 67% among controls (p < 0.01).
130
The INTERACT study was a randomized clinical trial in which 303 patients using BP
and/or lipid-lowering medications were randomized to receive or not receive text messages.
The group that received text messages improved medication adherence at 6 months compared
with the group that did not receive messages. The overall improvement in medication
adherence was 16%.
129,131
A Cochrane systematic review
132
sought to establish the effectiveness of mobile phone-based interventions in improving
adherence to medications prescribed for primary prevention of cardiovascular disease
in adults. The participants in the trials were recruited from community-based primary
care or outpatient clinics in high-income (Canada, Spain) and upper- to middle-income
countries (South Africa, China), but the interventions varied widely. One trial evaluated
an intervention focused on adherence to BP medication delivered exclusively by text
messaging, while another trial involved BP monitoring combined with feedback delivered
via smartphone. The authors considered the body of evidence for the effect of cell
phone-based interventions on objective outcomes (BP and cholesterol) having a low
quality. Of two studies that evaluated medication adherence along with other lifestyle
modifications, one reported a small beneficial effect on lowering low-density lipoprotein
cholesterol while the other found no benefit. Another trial (1,372 participants) on
an intervention based on text messaging showed a small reduction in systolic BP in
a group that delivered information-only text messages, but uncertain evidence of benefit
in a second intervention group that provided additional interactivity. One study examined
the effect of BP monitoring combined with smartphone text messaging and reported moderate
intervention benefits to systolic and diastolic BP. There was conflicting evidence
from trials targeting medication adherence along with lifestyle advice using multicomponent
interventions. Another study found large benefits on BP levels, while another study
showed no such effect. The authors of this Cochrane review concluded that there is
low-quality evidence related to the effects of interventions delivered via mobile
phone in increasing adherence to medications prescribed for primary prevention. The
conclusion based on this review is that there is current uncertainty about the effectiveness
of such interventions.
2.7.3. Emergency Services
Brazil has a geographically distributed health care system in which UBSs, emergency
care units (ECUs), secondary hospitals, and ambulances are scattered across the country,
often at remote locations. Specialized centers are located in advanced care units,
like tertiary hospitals, located in major cities. In this context, telemedicine tools
can improve emergency management.
133
Telemedicine has different applications in emergency services, ranging from electrocardiographic
transmissions associated or not with synchronous teleconsultations to assist in the
early diagnosis and management of cases of acute coronary syndrome (ACS); clinical
DSSs to help with the diagnosis, management, and prediction of cardiac complications
in patients with ACS;
133
transmission of bedside ultrasonographic images before hospital admissions;
134
and image transmission and support in the diagnosis and management of patients with
acute stroke.
135
The use of DSSs could increase the adherence to guideline recommendations in the management
of patients with ACS, but evidence on its impact on clinical outcomes in this context
is still limited.
135
2.7.4. In Systems of Care for Acute Myocardial Infarction
Systems of care for AMI integrate preadmission services, hospitals, and hemodynamic
services comprising the care of patients with AMI in a given region in order to optimize
the management of clinically suspected patients. The proposal of these systems is
to delineate the patients’ care flow involving early diagnosis, preadmission care,
initial treatment, use of thrombolytic agents, referral to a specialized hospital,
and post-event follow-up. They target high-quality, effective, safe care for patients
with AMI by optimizing resources and reducing disparities in their access to care.
133,136
Telemedicine services may play a crucial role in AMI systems of care, as they facilitate
the communication between a physician in an emergency unit, low-complexity hospital,
or pre-hospital admission with cardiologists at the hub or hospital with a hemodynamic
center that will receive the patient. Cardiologists can assist in (i) analyzing and
interpreting electrocardiograms for accurate and early diagnosis of ST-segment elevation
AMI,
132,137
(ii) guiding the best course of action, including the administration or not of thrombolytic
agents and other medications, through synchronous teleconsultations, i.e., real-time
communication between the on-site professional and the remote specialist,
137,138
and (iii) monitoring the patient’s clinical condition through telemonitoring, with
synchronous data transmission.
137
The incorporation of telemedicine strategies in systems of care for AMI is a worldwide
trend. A recent meta-analysis including studies conducted in Europe (11), North America
(8), South America (5), Asia (9), and Australia (2), with a total of 16,960 patients,
found consistent moderate-quality evidence that telemedicine strategies associated
with usual care in this context reduce in-hospital mortality by 37% (RR 0.63, 95%CI
0.55-0.72), with a number needed to treat (NNT) of 29 (95%CI 23-40) when compared
with usual care without telemedicine. The study also found poor quality evidence that
this intervention can reduce door-to-balloon time (mean difference 28 minutes, 95%CI
-35 to -20 minutes) and 30-day (RR 0.62, 95%CI 0.43-0.85) and long-term (RR 0.61 95%CI
0.40-0.92) mortality.
138
In Brazil, Belo Horizonte, Campinas, Salvador, São Paulo, and the Northern Region
of Minas Gerais (encompassing 89 municipalities) have published initiatives in this
area.
137,139-143
Decreased system delays and increased reperfusion rates have been observed in cases
of ST-segment elevation AMI, with evidence of reduced hospital mortality.
139,143,144
A typical telemedicine system comprises a specialized center (hub) and multiple remote
care units distributed within a geographic region (spoke centers) connected bidirectionally
by a communication channel. The specialized center may be a referral hospital in cardiology,
the operation center of an Emergency Mobile Care Service (Serviço de Atendimento Móvel
de Urgência, SAMU), or a telemedicine center. Some systems of care for AMI comprise
more than one specialized center, each with specific remote units for regional coverage.
145
The 2015 Telemecardiology Guideline for the Care of Patients with Acute Coronary Syndrome
and Other Heart Diseases details models of care using telemedicine systems for the
care of patients with ACS.
133
2.7.5. In Controlling the Use of Anticoagulants
The strategy of self-management of anticoagulants has been associated with a significantly
lower risk of ischemic stroke and all-cause mortality compared with direct treatment
with oral anticoagulants, while no significant differences were observed for major
bleeding and mortality. However, decreased surveillance is a potential problem for
the detection of patients who are unable to take care of their own treatment. A structured
education program is required for patients and/or caregivers and for involved professionals
in health care and quality control.
146-148
2.7.6. Cardiac Rehabilitation
Guidelines recommend that patients should undergo cardiac rehabilitation after AMI,
percutaneous coronary intervention (PCI), or myocardial revascularization. However,
rehabilitation is still underused, with participation of only 14-31% of all eligible
patients. Patients’ inability to attend the sessions and costs are important barriers.
149
Telehealth interventions using ICTs to enable remote rehabilitation programs can overcome
common barriers to rehabilitation access while preserving clinical supervision and
prescription of individualized exercise.
150
In a systematic review of 11 studies, the types of intervention were variable and
included the use of mobile or computer applications, biosensors, and interventions
delivered by landline phone lines. The interventions involved prescription and/or
monitoring of the participants’ performance and adherence. All interventions included
feedback, education, psychosocial support, and/or behavioral changes via landline
phone communications, mobile messaging, e-mail, website use, online tutorial, or online
chat.
151
The level of physical activity was higher in the intervention group compared with
the usual care group. Compared with face-to-face rehabilitation, the intervention
with telehealth was more effective in improving the level of physical activity, exercise
adherence, diastolic BP, and LDL-cholesterol, with poor- to moderate-quality evidence.
Telehealth rehabilitation was similar to face-to-face rehabilitation in maximal aerobic
exercise capacity and other modifiable cardiovascular risk factors.
151
The Telehab III study was a prospective, randomized, multicenter controlled trial
with patients undergoing cardiac rehabilitation. In all, 140 patients were randomized
to a conventional rehabilitation group or a 24-week Internet-based telerehabilitation
group associated with conventional rehabilitation. The additional telerehabilitation
program showed improvement in physical fitness and quality of life and induced persistent
health benefits.
152
A clinical trial conducted in China randomized 98 patients with NYHA I-III functional
class heart failure to an 8-week home-based teleconsultation exercise training program
or usual outpatient follow-up. Statistically significant improvements were observed
in the experimental group in terms of quality of life and result in the 6-minute walking
distance test compared with the control group. These results confirm that physical
training via teleconsultation is an effective alternative method for cardiac rehabilitation.
153
The noninferiority, randomized controlled trial REMOTE-CR tested the effects and costs
of cardiac rehabilitation by real-time teleconsultation in 162 patients with heart
failure and demonstrated this to be a cost-effective alternative that can enhance
the scope of rehabilitation.
154
Home-based cardiac rehabilitation is an alternative to increase patients’ engagement
in the program by presenting greater flexibility and activity options, offering choices
based on the patients’ values and preferences, and allowing the implementation according
to the patients’ daily routine.
155
The association of cardiac rehabilitation with conventional rehabilitation has been
shown to be more effective and efficient compared with a conventional rehabilitation
program alone, as it reduced the rates of readmission due to cardiovascular causes
and improved quality of life during the study period.
156
2.7.7. Remote Monitoring by Implantable Devices
Pacemaker telemonitoring has not significantly improved quality of life and number
of cardiovascular events, but provided early detection and treatment of events, reducing
hospital admissions and visits (routine and emergency) at lower costs compared with
hospital follow-up.
157
Implantable cardiac defibrillators (ICDs) or resynchronization defibrillators are
another type of implantable monitoring systems. Some of these devices may be equipped
with software for multiparametric monitoring of, for example, thoracic impedance and
right ventricular filling pressure with measurements captured by a right ventricular
lead. A groundbreaking study published in 2008 showed their clinical benefits in patients
with NYHA functional class III heart failure.
158
The IN-TIME trial later tested a similar strategy using multiparametric monitoring
devices (ICDs and resynchronization defibrillators). The parameters evaluated in the
trial included events like ventricular and atrial tachyarrhythmia, low percentage
of biventricular pacing, increased frequency of ventricular extrasystoles, decreased
patient activity, and abnormal intracardiac electrogram. Abnormalities in these parameters
triggered a structured phone contact. The group allocated to telemonitoring had a
significant reduction in combined clinical outcomes and total mortality.
159
Other similar studies have also shown a reduction in combined clinical outcomes, often
related to a decreased need for face-to-face visits.
160
Results from an unselected population cohort study also indicated benefits of remote
monitoring with information from ICD/cardiac resynchronization therapy (CRT) on mortality.
161
However, a meta-analysis
162
of 11 randomized trials evaluating 5,703 patients showed no consistent results on
clinical outcomes. The meta-analysis showed that device telemonitoring was associated
with a reduction in the total number of planned, unplanned, and emergency room visits
(RR 0.56, 95%CI 0.43-0.73, p < 0.001). However, rates of cardiac-related hospitalization
(RR 0.96, 95%CI 0.82-1.12, p = 0.60), the composite endpoints of emergency visits,
unplanned hospital visits, or hospitalizations (RR 0.99, 95%CI 0.68-1.43, p = 0.96),
and total and cardiac mortality were also similar between groups.
162
2.7.8. Atrial Fibrillation
Patients with atrial fibrillation (AFib) comprise a special group, considering that,
among other things, AFib has been accounted for approximately 60% of pacemaker and
CRT/defibrillator (CRT-Ds) alerts and nearly 10% of all ICD alerts in a worldwide
database.
163
Remote monitoring has excellent sensitivity (95%) in detecting AFib, a feature that
becomes even more important, considering that 90% of the detected episodes were asymptomatic.
164,165
The potential benefits of remote monitoring include detection and early reaction (e.g.,
drug therapy, device reprogramming, or electrical cardioversion) to prevent atrial
remodeling and serious adverse events related to AFib. The IMPACT trial has shown
that the detection of asymptomatic AFib via remote monitoring considerably shortened
the time to anticoagulation initiation (3 days versus 54 days) but was not associated
with reduced rates of stroke, systemic embolism, and bleeding.
166
In the preclinical phase of an arrhythmia, telemedicine screening may detect asymptomatic
AFib.
89
In a pilot study, the daily transmission of electrocardiographic data by phone facilitated
the diagnosis of asymptomatic paroxysmal AFib.
90
In large cohorts, telecardiology services improved the management of patients with
AFib and detected new cases of arrhythmia.
167
Support by telemedicine can improve the diagnosis of silent AFib.
168
Bilgi et al.
169
demonstrated that home-based electrocardiographic assessment supported by telecardiology
increased the sensitivity of the diagnosis of AFib in elderly individuals and was
useful in identifying individuals with AFib and atypical symptoms at home.
169
The electrocardiogram (ECG) was recorded and transmitted by a smartphone to a 24/7
telecardiology center and evaluated by a cardiologist. The telecardiology support
increased two times (40 years), four times (60 years), and seven times (70 years)
the rate of AFib diagnosed at home.
The SEARCH-AF study has shown that the use of an ECG lead (DI) on an iPhone ECG (iECG,
AliveCor KardiaMobile) accurately diagnosed AFib, making this technology feasible
for the screening of subclinical AFib in primary care and in the community.
170
In the REHEARSE-AF study, a randomized trial of AFib screening involving 1,001 participants
aged ≥ 65 years and with CHA
2
DS2-VASc ≥ 2,
171
the participants were randomized to screening with AliveCor KardiaMobile (iECG) twice
a week for 12 months (and additional ECG in case of symptoms) or usual routine. The
use of iECG increased by almost four times the diagnosis of AFib (HR 3.9, p = 0.007).
Smartphones, apps, and cloud storage technology have the potential to change the practice
of medicine and the way decisions are made. On smartphone platforms, medical or health
care applications can analyze a range of vital signs through built-in sensors, interconnected
devices, or peripherals.171 The transfer of ECG images by multimedia messaging can
be a practical, low-cost procedure in telecardiology.
172
These new technologies may increase the detection of arrhythmias, but the real value
of these new methods has yet to be evaluated in rigorously conducted studies.
2.7.9. Channelopathies
Inherited electrical syndromes are less frequent indications for ICD implantation.
However, their management can be challenging because these devices are then implanted
in patients who are often younger and less likely to comply with the required follow-up.
174
Electrical abnormalities may occur in these diseases, predisposing the patient to
unnecessary shocks and requiring careful programming.
175
The pediatric population, which often has implanted epicardial electrodes, is specifically
more vulnerable. In such patients, telemonitoring may be particularly useful for surveillance,
early detection, and preventive programming.
176
In the multicenter Brugada registry, the number of outpatient visits was significantly
lower in a telemonitoring group compared with a control group (p < 0.001), and there
was a trend suggesting that the number of inappropriate shocks was also reduced.
177
2.7.10. Tachycardia and Ventricular Fibrillation
Remote patient monitoring can be valuable for prompt assessment of the appropriateness
of the detection and the efficacy of the administered therapy. If shock is appropriate,
and clinical condition is stable, the physician can reassure the patient without requiring
a hospital visit. In a multicenter pilot study, 81% of the episodes of ventricular
tachyarrhythmia were analyzed remotely, and in 85% of the cases, no further action
was required.
178
The TRUST study demonstrated that remote monitoring allows early detection of ventricular
tachyarrhythmias compared with standard follow-up (1 day versus 36 days for ventricular
fibrillation and 1 day versus 28 days for ventricular tachycardia, p < 0.001).
179
Other potential benefits of remote monitoring include the prevention of inadequate
shocks and appropriate but unnecessary shocks. Inadequate detection due to supraventricular
tachyarrhythmias (or T-wave oversensing) may lead to the patient receiving a notification
for in-hospital reprogramming or other interventions.
180
Proper delivery of ICD shock for slow, stable ventricular tachycardia may lead to
device reprogramming with broader use of painless antitachycardia therapies. Recurrent
and self-limited asymptomatic rapid ventricular tachycardia occurring in the ventricular
fibrillation window (triggering alerts in some systems, regardless of the administered
therapy), can be detected early and, with appropriate intervention, be programmed
to prevent electrical storms. In addition, timely treatment of tachycardias may prevent
early battery depletion caused by recurrent loads and shock administration.
176,181
2.7.11. Congenital Heart Disease
Tele-echocardiography can establish an early diagnosis of congenital heart diseases
to guide therapeutic management.
182
A North American multicenter study in 338 paired infants (with and without access
to telemedicine) with or without minor heart disease showed a statistically significant
reduction in the percentage of infants transferred to a tertiary hospital (10% versus
5%), as well as in total hospital stay and intensive care unit (ICU) stay.
183
Telemedicine increases the ability of pediatric cardiologists to provide higher quality
care to a greater number of patients, although high-quality studies evaluating the
impact of this intervention are still limited.
2.8. Cardiovascular Teletomography and Teleresonance
Despite the apparent similarity between teleimaging and local diagnostic services,
divergences between both occur in one fifth of the cases. Specifically, divergences
with clinical impact occur in up to 1 to 3% of the cases. We can hypothesize that
the reasons for these divergences may be inadequate imaging quality, unavailability
of patients’ clinical data (like current and past history and physical examination),
limited access to patients’ laboratory tests and other imaging evaluations, fatigue,
and simple interobserver variation.
The workflow of imaging diagnosis in local hospitals and in telediagnosis may be difficult
to distinguish. Generated images are stored in imaging systems (picture archiving
and communication system - PACS, for example) and then analyzed by the radiology department
(and other specialties working with imaging tests, like cardiology and obstetrics).
In teleimaging, the image is transmitted to an external center and analyzed the same
way that it would be done locally. Further studies are needed to compare the diagnostic
performance of teleimaging versus local imaging. Both may even be assumed to have
the same performance, but evidence is critical to confirm our assumptions and can
determine not only whether teleimaging can be performed, but also the optimal conditions
to be carried out safely and cost-effectively without harm to the patient.
2.8.1. DICOM Standard
A new group of services developed in previous years was introduced in 1993, the Digital
Imaging and Communications in Medicine (DICOM) standard. The objective was to standardize
data and information obtained by imaging methods, normalizing the rules for transmission
and storage of medical information. This group of services uses a digital format that
associates images with metadata-type information with the ability to optimize search
and exchange of information and the use of images by specific software programs. DICOM
specifications are updated from time to time without losing sight of previously established
functionalities.
184,185
2.8.2. MRI, CT, and Telemedicine
Contrast-enhanced magnetic resonance imaging (MRI) and computed tomography (CT) scanning
bear additional complexities, requiring specific care at centers performing these
tests. These range from the scheduling of the tests - in which there is a need to
define its precise indication, e.g., pharmacological stress test (dipyridamole, adenosine,
dobutamine), evaluation of myocardial ischemia, viability, valvular heart disease,
specific cardiomyopathies, among others - to the need of on-site physicians due to
frequent use of contrast and medications, nursing technicians to obtain an adequate
venous access, and biomedical doctors and technologists with specific training and
experience in the acquisition and postprocessing of MRI and CT scan images using software
dedicated for these analyses.
The images should be read by experienced specialized physicians with specific training
in that particular area of diagnostic imaging. Such training usually requires 2 years
and is not widely available nationwide, limiting the number of trained specialists
for appropriate MRI and CT scanning.
The complexity of performing MRI and CT scans with remote guidance and reading, together
with the need for specialists to analyze the images and the possibility of the test
being performed at any given time, depending on the clinical indication, makes telemedicine
increasingly important for this activity.
2.8.3. The Federal Council of Medicine and Tele-CT/Tele-MRI
In 2014, the CFM updated the rules for tele-CT/tele-MRI practice in Brazil.
186
These rules are valid for the transmission of patients’ images between different locations
to produce a medical report, a second expert opinion, or a clinical imaging review.
The rules related to the topic of this document are listed below:
Clinical data - The transmission of tests by tele-CT/tele-MRI should be accompanied
by necessary clinical data of the patient, collected by the requesting physician,
for the preparation of the report.
Patient authorization - The patient must authorize on an informed consent form the
transmission of images and data.
Local and remote specialist - The responsibility for the remote transmission of tests
and reports must be assigned to a specialist in MRI and CT scanning.
Limits for remote practice - In the case of noncontrast imaging (e.g., calcium score
- CS), and in the absence of a specialist physician at the health care facility, the
attending physician may ask the specialist for appropriate remote diagnostic support.
Specialist required - A specialist physician must be present in centers where contrast
imaging tests - including MRI and CT scans - are performed.
Shared responsibility - The professional responsibility for the care lies with the
specialist physician caring for the patient undergoing the test. The specialist issuing
the remote report shares this responsibility.
Headquarters in Brazilian territory - Legal entities providing services in tele-CT/tele-MRI
must be headquartered in Brazilian territory and be registered with the CRM of their
jurisdiction. If the provider is an individual, he or she must be a physician trained
in MRI and CT scanning.
Operating standards - This is beyond the scope of this document, but specific information
can be found in another document on operating standards and minimum requirements for
the transmission and handling of remote imaging diagnostic reports.
Image compression and transmission - Communication protocols, file formats, and compression
algorithms should comply with current DICOM and HL7 standards. The specialist physician
is responsible for evaluating the compression ratio.
Image visualization and processing - The specialist physician is responsible for ensuring
the technical characteristics of remote workstations, monitors, and ergonomic conditions
in order to avoid compromising the diagnosis.
Safety and privacy - Computerized systems used for the transmission and handling of
clinical data and diagnostic imaging reports and for sharing of image and information
must comply with CFM regulations. Specifically, tele-CT/tele-MRI, systems must meet
the mandatory requirements of the “Level of Safety Assurance 2 (Nível de Garantia
de Segurança 2, NGS2)” established in the current Certification Manual for Electronic
Health Registration Systems issued by the CFM and the Brazilian Society of Health
Informatics (Sociedade Brasileira de Informática em Saúde, SBIS).
2.8.4. Imaging Transmission
Imaging transmission must comply with CFM standards regarding quality and security.
However, MRI and CT images are generally larger, requiring an infrastructure with
adequate data bandwidth for transmission. Importantly, the specialist physician responsible
for the report must download the images. Thus, the choice of important sequences after
image acquisition and the method of compression are fundamental for a smooth flow.
2.8.5. Postprocessing Software and Workstations
Ranging from ECG images to three-dimensional coronary angiotomography (TCA) images
and the wide variety of MRI sequences, several imaging parameters must be evaluated,
most requiring specific software.
Assessment of CS - CS is assessed with software programs installed in workstations,
which are usually purchased with the CT equipment, or with other independent programs
or plug-ins. The report usually informs the amount of calcification in each artery
along with the total CS value and percentile for the patient’s gender and age, based
on several population studies, of which the most used and recommended is the MESA
(MultiEthnic Study of Atherosclerosis) study.
187,188
Other data such as arterial age and the patient’s overall cardiovascular risk variation
based on the ECG can also be described.
Evaluation of TCA - After the appropriate acquisition phase, aiming at the best temporal
and spatial resolution of the coronary arteries, and editing of the ECG, which is
usually done directly in the CT equipment, other applications are helpful in establishing
a diagnosis.
Until the requesting physician is able to obtain a clear view, the interpretation
of the findings may be helped by software programs that extend the coronary arteries
in a single plane (curved planar reconstruction - CPR), visualization of three-dimensional
rendered images (using a 3D-volume-rendering technique), visualization of bidimensional
images with multiple oblique planes (multiplanar reconstruction - MPR), and the characterization
of coronary plaques, as well as the objective measurement of stenoses.
189
Evaluation with MRI - As one of the most versatile imaging methods available, MRI
is able to produce images of almost any anatomical plane and provide a wide range
of pulse sequences to generate images with specific characteristics, allowing assessments
that range from the evaluation of ventricular function to myocardial tissue characterization.
190
Several software applications are available for this purpose, including applications
for:
assessment of volume, ventricular mass, and right and left cardiac function;
analysis of intracardiac flow to measure QP-QS, shunts, and valvular dysfunction;
magnetic resonance angiography postprocessing with measurement of vascular diameters;
tissue characterization to quantify perfusion, necrotic/fibrotic mass, iron deposition
by T2* evaluation, and parametric maps of T1, T2, and T2* values.
The strategy of using software programs in MRI and CT imaging is strongly recommended
and can improve the time required to read the images, the accuracy of the reading,
and the clarity of the report of the findings.
2.8.6. Database, Communication, and Image Archiving
The integration between radiological information system (RIS) and PACS enables the
assignment of a unique registration for each patient. This optimizes the information
by combining images with clinical data and making the process faster and more secure.
This format has been increasingly used in health care centers and often enables remote
access, facilitating the use of tele-CT/tele-MRI and improving administrative procedures
and communication.
Several solutions are available in this regard, including cloud-based web solutions.
Remote access to images and the ability to distribute reports via a standard universal
system are helpful for the workflow.
A report is nothing more than a type of communication with the main objective of transmitting
the assessment of images analyzed by an expert to another physician who needs such
information to make decisions. The more complete and clear the transmitted information
is, the more important the requested test becomes. The development of structured reports
linking written information to tables, figures, and photos to make the information
as clear and accurate as possible is an ongoing trend.
As described earlier, reports may be made available through advanced systems like
RIS, but other forms of transmission, including instant messaging applications like
WhatsApp, may be used. According to the CFM,
191
WhatsApp and similar platforms can be used for communication between physicians and
patients, as well as privately between physicians for transmission of data or questions,
or in closed group chats between specialists or clinical staff of an institution or
chair, provided that all information transmitted is absolutely confidential, remain
within the group, and is not circulated to recreational groups, even if these are
composed only of physicians.
2.8.7. Clinical Indications for MRI and CT
Interestingly, no studies in the literature have assessed the clinical impact of the
application of tele-MRI or tele-CT. Thus, clinical recommendations in this guideline
are based on level C evidence, including expert consensuses, and in the absence of
studies evaluating cost-effective outcomes. Aware of this limitation, we cite at the
end of this document the main indications for the application of tele-CT/tele-MRI
in this subarea of cardiovascular imaging.
192
The use of MRI and CT imaging has been increasing, and characteristics of these imaging
methods make their use very interesting in telemedicine, particularly in countries
with continental proportions like Brazil and in those with a limited number of available
MRI/CT specialists. The possibility of having a specialist potentially accessible
at any moment can be helpful in patient management and in lowering health care costs
by optimizing the time of available specialists and expediting reports of hospitalized
patients, which can shorten their hospital stay, and in other applications related
to this medical progress.
3. Telerobotics Applied to Cardiology
3.1. Robotic Telesurgery
The concept of telesurgery was introduced in the early 1970s by NASA.
193
The objective of the original project was to provide medical care to astronauts during
remote missions.
Robotic telesurgery devices are applications in which the surgeon controls remotely
a robot that executes the surgical procedure. The da Vinci® system (da Vinci® surgical
system; Intuitive Surgical, Sunnyvale, CA, USA), the most widely used robotics platform
today, follows this approach. The surgeon works on a console separated from the surgical
field, and the movement of his or her hands is perceived and transmitted to the instruments
close to the patient. This technique yields great ergonomic benefit to the surgeon,
incorporates functions like hand tremor cancellation, and broadens (in three dimensions)
the view of the field that the surgeon is interested in. However, these platforms
lack much automation and require continuous involvement of a human operator (surgeon)
for regulatory reasons.
ARTEMIS was the first surgical robot used for cardiac procedures.
194
Designed as a telesurgery and telepresence system for cardiac procedures, it was used
for training and planning and to perform minimally invasive procedures.
Currently, robotic cardiac surgery has been used primarily for mitral valve repair
and myocardial revascularization, following approval by the Food and Drug Administration
(FDA) in 2002 and 2000, respectively. Newer techniques assist in cardiac manipulation
procedures by compensating heart movements. However, large-scale implementation of
this technique is hampered by its high cost
195
and the absence of randomized studies demonstrating its superiority over traditional
minimally invasive techniques, with or without hybrid procedures.
196
The first robotic mitral valve heart surgery was performed in 1998 by Carpentier,
in France, and Mohr, in Germany. That same year, Carpentier conducted the first coronary
artery bypass surgery in Paris, while Reichenspurner performed revascularization surgery
with the voice-controlled ZEUS Robotic Surgical System (Computer Motion, Goleta, CA)
in Munich.
196,197
Since then, this technique has become popular as it is associated with less surgical
aggression, reduced cardiopulmonary bypass and aortic clamping duration, and shorter
hospital stay compared with the conventional open technique.
197
The da Vinci® robotic system has allowed improved visualization of the surgical field
with three-dimensional capture and ten-fold magnification, resulting in greater precision
in the surgical procedure and smaller incisions following a learning curve.
198,199
It has also reduced the rates of all complications (particularly infection), blood
transfusion, and time to return to work activities, with an impact on the total costs
of the procedure. This has been observed especially among patients at high risk (like
elderly individuals) and those with ejection fraction lower than 20%, diabetes of
difficult control, and severe chronic obstructive pulmonary disease.
200
This robotics system has also benefited hybrid surgeries, angioplasty, and minimally
invasive procedures in patients with multilateral obstructive coronary artery disease.
201
Both computer-integrated surgery and telemedicine are becoming popular in the developed
world. Advancements in robotic technology and information technology, like the Internet
of Things, allow robotic arms to be controlled remotely, enabling robotic telesurgery.
With telesurgery, surgeons can perform surgical procedures in remote locations, away
from the patients, improving the access to medical treatment and, potentially, the
quality of the treatment.
As with other telemedicine applications, telesurgery can broaden the access to interventions
in remote areas or centers where specialists in particular types of surgery are not
present, for example. The importance of telemedicine, telesurgery, and remote surgery
is not restricted to their ability to perform medical procedures in areas where these
procedures are not available and can be extended to telementoring, which involves
the training of medical professionals to perform these procedures.
202
In this area, telesurgery could also benefit patients requiring infrequent, highly
complex interventions, in which the medical-surgical expertise is not widely available.
The acquisition of new technology expertise by specialists has been accompanied by
mentoring programs (proctors). In robotic surgery, telemedicine can provide training
with remotely connected proctors (telementoring), expanding the access to innovative
technologies.
203,204
Outcomes of robotic surgeries still lack long-term analyses of hard outcomes like
all-cause mortality, cardiovascular death, fatal AMI, stroke, need for repeat revascularization,
and vascular graft patency. As in traditional surgeries, patient selection is essential,
and the goal should be complete revascularization, bearing in mind that CO2 insufflation
decreases venous return by increasing intrathoracic pressure and may compromise hemodynamic
parameters in patients with left ventricular dysfunction and in those with chronic
obstructive disease, who would benefit more from minimally invasive surgery. Case
series have been reported totaling about 110 patients and showing 90% of surgical
success within 30 days without open surgery and a maximum follow-up of 5 years.
205-207
The largest experience in this area has been published by Cavallaro et al.,
208
who reported rates of morbidity and mortality with robotic myocardial revascularization
surgery in 2,582 patients between 2008 and 2010. The authors reported lower rates
of postoperative complications in selected patients but concluded that these benefits
decreased in patients requiring multiple bypass grafts.
208
Approximately 1,700 robotic heart surgeries are performed annually in the US, including
35.5% for mitral valve repairs.
209
In 2011, the FDA introduced a post-marketing surveillance plan known as the Medical
Device Epidemiology Network initiative, leveraging AI to real-world data analysis,
including international registries and electronic medical record data, to bridge the
gap in evidence gathering concurrent with the implementation of technological innovation
without compromising patient safety.
210
In Brazil, none of the robotic surgical techniques have been included in the public
policy list or in the List of Procedures and Events in Health of the National Health
Agency.
210
In this sense, the CFM, through Resolution 2.227/2018, which was later repealed, had
regulated robotic telesurgery, precisely anticipating the expansion of the benefits
of the technique and facilitating the follow-up of the learning curve by proctors
in remote locations. Thus, robotic surgery in Brazil can be remotely assisted by a
specialist at a large center in another country. Although the CFM allowed the use
of robotic telesurgery in Brazil, it restricted the method to professionals qualified
to practice medicine in the country.
39
Of note, mentoring/proctoring programs in Brazil have not been widely regulated, except
in the State of Paraíba, where the CRM, through Resolution CRM-PB 182/2018, defined
rules that regulate and legally secure the practice to the medical act.
39
In addition to the information presented above, synchronization between the vast potential
of these technologies and the existing ethical and legal apparatus is also lacking.
Unlike a comprehensive national policy, there is a general scenario of fragmentation
characterized by different norms and standards issued by different agencies and with
a different focus.
7
Even if a single instrument could hardly achieve these objectives, fragmentation would
be yet another obstacle to overcome to reach the potential that telemedicine and telesurgery
have in our country.
Among other barriers to their practical use are the scarcity of resources and technical
knowledge, as well as infrastructure issues. Brazil is a country of unequal regional
distribution in terms of broadband availability.
7
This means that the infrastructure of the broadband data network is one of the most
limiting factors for the expansion of telemedicine in general and telesurgery in particular,
especially in rural areas of the country.
3.2. Robotic Angioplasty
PCI can be considered a highly predictable, safe, and minimally invasive therapy.
However, this manual and operator-dependent procedure must be executed in person,
demanding physical action by the physician. Full proficiency can only be achieved
in high-volume environments with scenarios involving highly complex and technological
interventions. Coronary angioplasty also exposes both professional and patient to
ionizing radiation. As a result, the potential risks are high for occupational health
damage arising not only from the radiation but also from the need of individual protection
211-213
e.g., a 7-kg lead apron).
Robot-assisted coronary interventions have recently been developed as an alternative
to reduce the reliance on manual operation,
214-224
potentially reducing damage from radiation exposure.
225
Clinical studies have demonstrated the safety and efficacy of the robotic system,
which has already been approved for routine application in the US and the European
Union. Even though the current set of scientific evidence is encouraging, it is also
recent and limited to the number of patients treated, hindering further consideration
of possible risks and benefits of the technique, especially when it comes to particularities
of its application in subgroups of clinical interest.
4. Telemedicine for Provision of Services and in Supplementary Health
4.1. Provision of Services
Over 60% of all health care facilities and between 40-50% of all US hospitals are
currently estimated to use some form of digital data transmission.
226
In 2016, a US health care facility reported that communication of digital health data
(e-mail, phone, and video) exceeded the number of in-person consultations.
227
However, it is important to note that despite new modes and means of communication
transmission between physicians and patients, ethical and legal responsibilities remain
the same as those governing the traditional physician-patient relationship.
228
Cardiologists must inform their patients about telemedicine services and their limitations,
the possibility of late follow-up, by encouraging regular reporting to their attending
physicians, and how they can receive electronic health-promoting information.
Reimbursement is a key determinant of the success of clinical interventions. The movement
toward value-based reimbursement rather than payment for service, which provides incentives
for care in lower-cost settings, along with the identification and interaction with
high-risk individuals before disease onset, and the efficient use of integrated care
teams, provide incentives for telemedicine expansion. Understanding the effect of
reimbursement within the context of alternative payment models is a priority.
While the path of value-based reimbursement is uncertain, the efficiency of care will
inevitably be a priority in any scenario. Ensuring that these technologies are used
for patients who meet the appropriate clinical requirements is also an important related
topic. In the US, reimbursement for medical services by telemedicine has gradually
expanded from coverage of services provided in rural settings to a broader program
(Medicare Access and CHIP Reauthorization Act).
229
Training and development projects have been created in Brazil, along with a continuing
medical education with special attention to the SAMU/UPA care model, developed by
the Ministry of Health and private hospitals.
230
Regarding the payment for telemedicine and telehealth services in Brazil, as already
mentioned, it should be considered that the main source of funds has been the public
sector:
231-233
public communications from national (such as the National Council for Scientific and
Technological Development - CNPq - and the Financier of Studies and Projects - FINEP)
and regional research and innovation funding agencies (state research supporting foundations);
agreements or direct transfer of funds to universities and health departments, within
the scope of the program Telessaúde Brasil, then Telessaúde Brasil Redes;
service providing agreements between public administrators and telehealth centers
at university centers;
232
projects within hospitals receiving tax waiving by the PRO-ADI SUS program from the
Ministry of Health.
215
Many of these investments occurred at an early stage of technological development
in the country, and only some of the fostered nuclei became active, sustainable services.
232
There is a clear need for inclusion of telehealth procedures in the list of procedures
paid by the SUS in order to regulate and encourage their routine use in the health
system.
Supplementary health, in turn, lacks formal mechanisms of payment and reimbursement
for telehealth activities, so telemedicine actions in this sector have been focused
on optimizing care and reducing costs, and are often associated with specific conditions
like stroke.
231
Of note, the Ministry of Health Secretariat of Science, Technology, and Strategic
Inputs, through Ordinance 26, of August 2, 2017, made public the decision to incorporate
remote monitoring technology for the evaluation of patients with cardiac implantable
electronic devices (CIEDs) within the scope of the SUS. This is an unprecedented incorporation
of remote monitoring technology as a result of industry demand. The requester assessed
the budgetary impact of the technology over 5 years, considering only direct expenses
with the purchase of the remote monitoring device and the provision of conventional
monitoring in a base case. A second scenario analyzed a model of dynamic transition
state to account for opportunity costs of both technologies, exploring the advantages
and disadvantages of each strategy. With the decision, the technology should have
been included in the SUS within 180 days from the publication of the incorporation
order by the Secretary of Science, Technology, and Strategic Inputs, but to date,
the Clinical Protocol and Therapeutic Guidelines making this technology available
in the SUS have still not been published.
234
Unfortunately, there are still several gaps in the process of recognizing medical
services in telemedicine for the purpose of reimbursement. The example above is the
only one deliberated for public health. The process of coding hierarchical classifications
or other payment modalities for the various telemedicine services has not yet been
properly structured.
A more complete and structured set of codes would also provide more accurate data
to address the scarcity of systematic economic evaluation of the benefits of telemedicine
in both pay-per-service and value-based models.
235
Bridging this gap is essential to guiding public and private health care providers
and technology buyers and investors on decisions about investment returns in this
field.
4.2. Telemedicine in Supplementary Health
In 1988, Brazil opted for the establishment of a universal health care system free
and fully accessible, pursuant to art. 196 of the Federal Constitution. However, health
care is available to private initiative, as established in the Magna Carta (Paragraph
1 of Art. 199) pertaining to the scope of participation of the private initiative:
“Private institutions may participate in a complementary way in the SUS, according
to its guidelines, by means of a contract of public law or agreement, with preference
given to philanthropic and nonprofit entities.”
236
Supplementary health assists over 47 million beneficiaries in Brazil and is governed
by its own legislation, regulated by the National Health Agency. This is a health
care system with its own characteristics and regulatory framework guided by specific
legislation (Law No. 9.656/1998). The benefits gained from the remarkable development
of ICT also apply to supplementary health. However, it should be noted that, due to
particularities of laws governing the sector, health care plan operators are required
to offer a myriad of procedures included in the List of Procedures and Events in Health
of the National Health Agency.
237
Also worthy of note is the fact that when one buys a health care plan, priority is
given to access to physicians and therapies that supplement the list of policies offered
by the SUS. Most of the supplementary health beneficiaries live in large centers,
with over 35 million beneficiaries in the Southeast (28,650,281) and South (6,912,748)
regions and more than 18 million in capital cities. An analysis of the intersection
of demographic data of supplementary health beneficiaries and Brazilian physicians,
including cardiologists, shows that the proportion of physicians per inhabitant (beneficiaries
of supplementary health) in these regions is different from the one observed in public
health, where truly remote areas receive no coverage. It is imperative to consider
that the in-person relationship between physician and patient is the rule in supplementary
health, which does not hinder the possibility of making telemedicine resources available.
238
Accordingly, the various services provided by telemedicine are fully applicable to
supplementary health. However, the provision of face-to-face consultations with experts
rather than their “tele” versions is a legal demand. ICT resources should not be offered
to replace face-to-face interaction but should be an option to improve care also in
the context of supplementary health.
Increased efficiency in health care requires quality improvements and costs savings.
239
The integration of telemedicine into outpatient clinics and hospitals, including supplementary
health, can help achieve both goals.
240
Medical care is, without question, one of the most (if not the most) complex sectors
in the economy. Considerable financial investment and years of persistence are required
to build an effective telemedicine system. The widespread adoption of this type of
practice also requires behavioral adaptations by many physicians, organizations, and
patients.
241
The industry in this area still requires better regulation.
Telemedicine can be an affordable alternative to meet the health needs of vulnerable
populations with multiple comorbidities requiring frequent care.
242
Improving patient engagement, telemedicine can provide an effective platform for patients
to engage in their own decisions.
243
For example, the US Veterans Health Administration introduced a national telemedicine
program named Care Coordination/Home Telehealth. This model allows patients to manage
their own conditions,
244
and some important studies have reported that the shared-decision model has reduced
hospitalization rates.
245
Unequal access to health, even in supplementary health, is persistent in Brazil and
requires major investments to improve the organization of health care systems. Even
when health care services and evidence-based guidelines are available for common and
relevant conditions like hypertension and diabetes, the implementation gap is vast,
and best practices are not absorbed by health care professionals, or recommended measures
are not followed by patients and their relatives. The science of implementation has
proved to be as important as data analysis in recent decades for the recognition of
bottlenecks preventing full use of preventive and therapeutic measures to ensure benefits
for patients who can live longer and better by taking advantage of all available knowledge,
246
reducing costs and increasing the efficiency of private health care systems.
Direct physician-patient teleconsultation, not yet regulated in Brazil, is the most
frequently used model. A US report showed that telecardiology was useful for evaluating
both new and recent postoperative referrals, with the potential benefit of knowledge
transfer to local primary care. In Canada, teleconsultation has been useful for the
evaluation of new patients with syncope and supraventricular tachycardia.
247
In the United Kingdom, a wide range of inpatient and outpatient telecardiology services
is available at district hospitals using various technologies.
248
This approach has improved access, was cost neutral, and increased patient satisfaction.
The authors emphasized that it complemented but did not replace regular consultations.
The demand for home care using web-based applications directed to consumers, including
tablet and smartphone applications, is growing exponentially.
249
Many health care providers worldwide are adopting this technology as a way to provide
low-cost care for common problems that could result in a visit to the emergency department.
250
Most of these applications rely solely on video and audio connections with additional
software for scheduling, billing, sharing of still images, and documentation. Also,
some peripherals, such as smartphone-compatible heart rate sensing devices,
251
are available for purchase at decreasing prices.
Limited financial availability for the acquisition and maintenance of telemedicine
equipment and infrastructure remains a barrier to the widespread deployment of telemedicine.
252
This is particularly true for many health care providers or small systems that may
lack the required resources and often have conflicting demands for available funds.
Other costs associated with telemedicine programs include salary, administrative support
and supplies, software and application development and upgrade costs, training programs,
and initiatives to promote the program to patients.
253
With increased mobile connectivity, smartphones, and video compression, the costs
to implement simple telemedicine interactions have decreased.
Currently, none of the procedures used in telemedicine are included in the Supplementary
Health List of Procedures and Events in Health, i.e., a regulatory vacuum current
places telemedicine in a field of conjecture and frustrated expectations dissociated
from the interest of the regulated sector. There is also no concrete scientific evidence
available to support the formal use of this technology.
38
Telemedicine is a disruptive innovation with the potential to change health, and its
influence is likely to increase rapidly. Guided by what is best for patients, telemedicine,
if properly applied, will help usher in a new age for health care that will be built
by patients and physicians, identifying new ways of care, increasing quality and rationalizing
costs also in supplementary care.
240
5. Economic Evaluation and Budgetary Impact of the Incorporation of Telemedicine in
Cardiology in Brazil
5.1. Concepts of Economic Evaluation in Telemedicine
The implementation of telemedicine services seeks to provide accessible and quality
health care at a low cost.
254
Since the 1990s, as technological communication capabilities advanced, telemedicine
services became more prevalent.
255
The emergence of new telemedicine-related capabilities and their integration into
care systems offers opportunities to enhance value-based clinical care, promote health,
and prevent diseases.
256
The values associated with the adoption of telemedicine services include the collaboration
with the agile accessibility of patients to highly complex centers,
257
along with a reduction in mortality
258
and frequency of hospitalizations.
259
In cardiology, recent publications portray telemedicine and teleconsultation services
as effective in the management of patients with chronic heart failure,
260,261
preparation of ECG reports, guidance of patients through mobile applications,
262
and cardiac rehabilitation,
263
among others.
From reported experiences, telemedicine is universally seen as offering interesting
benefits for improving accessibility and quality of health.
264
However, scientific and financial investments required to introduce these technologies
into the health care system are high,
265
which potentiates the importance of accurate studies on economic analysis to guide
decisions upon implementation of telemedicine services.
265,266
Economic analysis is one of the pillars of health care assessment that aims to support
and guide decision making. For a new technology to be applied to a process, it must
replace existing alternatives with equal or better results. That is, it must be effective.
In addition, the results thus obtained should be less expensive than the alternatives
or present reasonable values related to benefits, i.e., it must be cost effective.
However, the economic evaluation of telemedicine strategies has some specificities:
constant change of technology, lack of an adequate study design to manage undersized
samples, inadequacy of conventional economic assessment techniques, and health outcome
assessment problems not directly related to health.
267
As a consequence, different types of cost analysis have gained an important role in
the evaluation of telemedicine services.
5.2. Applied Economic Methods
Different approaches have been used to assess the economic impact of telemedicine
services with varying levels of acceptability. Basically, five methods are available
for calculation of cost effectiveness between conventional and telemedicine interventions:
cost-minimization analysis, cost-benefit analysis, cost-effectiveness analysis, and
cost-utility analysis. Additionally, return on investment has been used in telemedicine
projects.
268
Cost-minimization analyses assume that both alternatives (conventional and telemedicine)
are equally effective in health outcomes but differ in cost. A cost-minimization analysis
considers changes but keeps health outcomes unaltered. For example, telecardiology
for ECG reports assumes the same result but with different costs. Consequently, managers
have to consider only differences in costs when deciding which alternative is less
expensive.
269
Cost-benefit analysis recognizes that different projects are equally effective, but
results and costs change. Changes in health costs and outcomes are considered simultaneously
and attribute a monetary or numerical value not only to costs but also to health outcomes
in order to express the nondimensional cost/benefit ratio. However, the idea of attributing
monetary values to health outcomes, such as years of life, is not always acceptable
to health decision makers.
Cost-effectiveness analysis appears as a solution when costs and results are considered
simultaneously, without attributing monetary values to results. Each outcome is defined
according to its specific unit so that the final indices demonstrate a relationship
between economic and health outcomes. Therefore, the final decision depends on the
relationship that the decision maker considers best. This ratio (incremental cost-effectiveness
ratio, ICER) represents the cost for each additional result unit.
252
Cost-utility analyses consider individuals’ quality of life and the time of life that
they will obtain as a result of an intervention. This is a particular case of cost
effectiveness in which results are measured in terms of full-life lived years, usually
expressed in quality-adjusted life-years (QALYs) or disability-adjusted life-years
(DALY). The WHO recommends a value for DALY equivalent to three times the gross domestic
product per capita.
Return on investment is a nondimensional relationship between the monetary value invested
and the monetary gain resulting from these investments, and measures the efficiency
of the investment.
270
5.3. Literature Review
A systematic literature review of cost-benefit review studies on the adoption of telemedicine
services was conducted by the Federal University of Minas Gerais (UFMG) in 2016, gathering
publications from 2000 to 2016.
105
Considering the keywords employed in the study, a search was performed on the PubMed
database with the inclusion criterion being only studies related to cardiology. Other
reviews manually searched in databases and/or journals of the area were also added.
Thus, the search included literature review articles on the assessment of the incorporation
of telemedicine in cardiology from 2000 to April 2019.
Variables related to the economic analysis method, purpose of telemedicine in cardiology,
clinical effectiveness, and cost reduction were extracted from the studies. For effectiveness,
the impact on the reduction of mortality and hospitalizations, improvement of medication
management, and anticipation of diagnosis were evaluated.
From the search and application of exclusion criteria, 35 articles were fully analyzed
by two researchers (A.E. and B.Z.) for the collection of variables. Figure 5.1 presents
the flowchart of the selection of the articles.
Figure 5.1
Flowchart of selection of articles on economic analysis of Telemedicine applied to
cardiology.
The number of studies focusing on telemedicine, especially the monitoring of chronic
diseases, has increased over the last decade. Of the 35 studies identified, 19 were
published after 2015. Table 5.1 summarizes the characteristics of the articles.
Table 5.1
Studies on economic evaluations of telemedicine in cardiology
#ID
Authors
Year
Number of papers assessed
Category of telemedicine services
Service focus
Reduction in mortality
Reduction in hospitalizations
Reduction in hospital stay
1
López-Villegas A, Catalán-Matamoros D, Martín-Saborido C, Villegas-Tripiana I, Robles-Musso
E.
284
2016
7
Telemonitoring (mHealth)
Heart failure
N/A
N/A
Yes
2
Cajita MI, Gleason KT, Han HR.
271
2016
10
Telemonitoring (mHealth)
Heart failure
N/A
N/A
N/A
3
Louis AA Turner T Gretton M Baksh A Cleland JG.
270
2003
24
Telemonitoring (mHealth)
Heart failure
Yes
Yes
Yes
4
Kassavou A, Sutton S.
254
2018
17
Mobile and automated communication
Guidance on medication use
N/A
N/A
N/A
5
Lin MH, Yuan WL, Huang TC, Zhang HF, Mai JT, Wang JF.
264
2017
39
Telemonitoring (mHealth)
Chronic heart failure
Yes
Yes
Yes
6
Kotb A Cameron C Hsieh S Wells G.
285
2015
30
Comparison between telephone teleconsultation services
Heart failure
Yes
Yes
Yes
7
Yun JE, Park JE, Park HY, Lee HY, Park DA.
286
2018
37
Telemonitoring (mHealth)
Heart failure
Yes
Yes
N/A
8
Seto E.
269
2008
9
Telemonitoring (mHealth)
Heart failure
N/A
N/A
N/A
9
Grustam AS, Severens JL, van Nijnatten J, Koymans R, Vrijhoef HJ.
260
2014
22
Telemedicine
Chronic heart failure
Yes
Yes
N/A
10
Lee M, Wang M, Liu J, Holbrook A.
287
2018
7
Teleconsulting
Guidance on medication use
Yes
N/A
N/A
11
Klersy C, De Silvestri A, Gabutti G, Raisaro A, Curti M, Regoli F, et al.
288
2011
17
Telemonitoring (mHealth)
Heart failure
N/A
Sim
Não
12
Conway A Inglis SC Clark RA.;
289
2014
25
Telemonitoring (mHealth)
Hypertension
Yes
Yes
Yes
13
Duan Y, Xie Z, Dong F, Wu Z, Lin Z, Sun N, Xu J.
290
2017
46
Telemonitoring (mHealth)
Hypertension
N/A
Yes
N/A
14
Kitsiou S Paré G Jaana M.;
291
2015
15
Telemonitoring (mHealth)
Chronic heart failure
Yes
N/A
N/A
#ID
Reduction in hospital stay
Early diagnosis
Increased adherence to therapy
Not conclusive
Cost reduction
Most frequent economic analysis method among the articles
1
Yes
Yes
N/A
N/A
Yes
Cost-effectiveness
2
N/A
N/A
N/A
Yes
N/A
N/A
3
Yes
Yes
N/A
N/A
Yes
Cost-effectiveness
4
N/A
N/A
Yes
N/A
N/A
N/A
5
Yes
N/A
N/A
N/A
N/A
N/A
6
Yes
N/A
N/A
N/A
N/A
N/A
7
N/A
N/A
Yes
N/A
N/A
N/A
8
N/A
N/A
N/A
N/A
Yes
Cost-minimization
9
N/A
N/A
N/A
N/A
Yes
Cost-effectiveness
10
N/A
Yes
N/A
N/A
N/A
N/A
11
No
N/A
N/A
N/A
Yes
Cost-effectiveness
12
Yes
N/A
N/A
N/A
N/A
N/A
13
N/A
Yes
N/A
N/A
N/A
N/A
14
N/A
N/A
N/A
N/A
Yes
N/A
15
Polisena J Tran K Cimon K Hutton B McGill S Palmer K
292
2010
21
Telemonitoring (mHealth)
Congestive heart failure
Yes
Yes
Yes
16
Pandor A Thokala P Gomersall T Baalbaki H Stevens JW Wang J.
266
2013
2
Telemonitoring (mHealth)
Heart failure
Yes
Yes
N/A
17
Inglis SC Conway A Clark RA.; Cleland JG
285
2015
27
Teleconsulting
Heart failure
Yes
Yes
N/A
18
Liu S, Feng W, Chhatbar PY, Liu Y, Ji X, Ovbiagele B.
286
2015
13
Telemonitoring (mHealth)
Risk of stroke
Yes
N/A
N/A
19
Knox L, Rahman RJ, Beedie C.
287
2017
26
Telemonitoring (mHealth)
Chronic heart failure
Yes
Yes
Yes
20
Pandor A Gomersall T Stevens JW Wang J Al-Mohammad A Bakhai A.
288
2013
21
Telemonitoring (mHealth)
Heart failure
N/A
Yes
N/A
21
Hamilton SJ, Mills B, Birch EM, Thompson SC.
255
2018
9
Telemonitoring (mHealth)
Cardiac rehabilitation and heart failure
N/A
N/A
N/A
22
Inglis SC Clark RA Dierckx R Prieto-Merino D Cleland JG.
289
2015
25
Telemonitoring (mHealth)
Heart failure
Yes
Yes
N/A
23
Paré G Jaana M Sicotte C.;
290
2007
16
Telemonitoring (mHealth)
Hypertension and heart disease
N/A
Yes
Yes
24
Shah A Clarke M Sharma U.;
291
2011
13
Telemonitoring (mHealth)
Congestive heart failure
N/A
No
No
25
Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R.
151
2016
11
Teleconsulting
Cardiac rehabilitation
Yes
N/A
N/A
26
Neubeck L Redfern J Fernandez R Briffa T Bauman A.
292
2009
11
Telemonitoring (mHealth)
Coronary disease
Yes
N/A
N/A
#ID
Early diagnosis
Increased adherence to therapy
Not conclusive
Cost reduction
Most frequent economic analysis method among articles
15
N/A
N/A
N/A
N/A
N/A
16
N/A
N/A
N/A
Yes
Cost-effectiveness
17
N/A
N/A
N/A
N/A
N/A
18
Yes
NA
NA
N/A
N/A
19
Yes
N/A
N/A
N/A
N/A
20
N/A
N/A
N/A
N/A
N/A
21
Yes
Yes
N/A
N/A
Cost-effectiveness
22
Yes
N/A
N/A
No
N/A
23
Yes
N/A
N/A
No
N/A
24
NA
N/A
N/A
Yes
N/A
25
Yes
N/A
N/A
N/A
N/A
26
Yes
N/A
N/A
Yes
N/A
27
Huang K Liu W He D Huang B Xiao D Peng Y.
301
2015
9
Teleconsulting
Coronary disease
Yes
NA
NA
28
Purcell R McInnes S Halcomb EJ.
302
2014
13
Telemonitoring (mHealth)
Chronic heart failure
Yes
Yes
NA
29
Chaudhry SI Phillips CO Stewart SS Riegel B Mattera JA Jerant AF Krumholz HM.
303
2007
9
Telemonitoring (mHealth)
Heart failure
NA
Yes
Yes
30
Pfaeffli Dale L, Dobson R, Whittaker R, Maddison R.
304
2016
7
Telemonitoring (mHealth)
Cardiovascular diseases
NA
NA
NA
31
Rush KL Hatt L Janke R Burton L Ferrier M Tetrault M.;
262
2018
16
Tele-education
Chronic heart failure
NA
Yes
Yes
32
Hameed AS, Sauermann S, Schreier G.
305
2014
9
Comparison between telephone teleconsultation services
Heart failure
NA
NA
NA
33
Feltner C Jones CD Feltner C Zheng ZJ Sueta CA Coker-Schwimmer EJ Arvanitis M.
259
2014
47
Telemonitoring (mHealth)
Heart failure
NA
Yes
NA
34
Beatty AL, Fukuoka Y, Whooley MA.
150
2013
3
Telemonitoring (mHealth)
Cardiac rehabilitation
NA
NA
NA
35
Driscoll A, Meagher S, Kennedy R, Hay M, Banerji J, Campbell D, Cox N, Gascard D,
Hare D, Page K, Nadurata V, Sanders R, Patsamanis H.
258
2016
29
Telemonitoring (mHealth)
Heart failure
Yes
Yes
Yes
#ID
Early diagnosis
Increased adherence to therapy
Not conclusive
Cost reduction
Most frequent economic analysis method among the articles
27
Yes
N/A
N/A
N/A
N/A
28
Yes
N/A
N/A
Yes
N/A
29
N/A
N/A
N/A
Yes
Cost-effectiveness
30
N/A
Yes
N/A
Yes
N/A
31
Yes
Yes
N/A
Yes
N/A
32
Yes
Yes
N/A
No
N/A
33
N/A
N/A
N/A
N/A
N/A
34
Yes
Yes
N/A
N/A
N/A
35
N/A
N/A
N/A
N/A
N/A
Among the studies, 26 evaluated the telemedicine telemonitoring service mHealth as
primary intervention versus usual face-to-face treatment. According to the authors,
the ease of use of mobile phones, through the use of applications for remote monitoring
and quick communication with patients, was the most common.
271
The most common outcomes in the evaluated studies were a reduction in mortality rate
(54% of the reviews) and in the number of hospital readmissions (57% of the reviews),
with at least one of these outcomes appearing in 26 studies. Other expected results
were reduced hospitalization, early diagnosis, and better medication adherence.
On the other hand, economic evaluations were less frequent. Only 16 reviews found
conclusive results with cost analyses, of which 13 concluded that telemedicine provided
savings to the paying source by reducing costs. Eight reviews included the economic
evaluation of incorporating telemedicine in cardiology, with cost effectiveness being
the most frequent evaluation (seven studies), while cost minimization was included
in a single study. One study suggested that the few existing economic assessments
have low methodological rigor, not allowing an assertive conclusion about the economic
viability of the implementation.
264,271
Briefly, studies on heart failure telemonitoring have shown that support strategies
(video conferencing or telephone) are cost effective, meaning, they have a potential
for financial return. Studies evaluating monitoring by cardiac devices showed an incremental
cost-effective ratio of US$ 13,979 per QALYs. In a meta-analysis, device telemonitoring
was related to a 44% reduction in hospital visits, with no effect on mortality, but
a 15-50% reduction in direct health costs.
272
The economic results of noninvasive telemonitoring are even more heterogeneous. Some
clinical trials have shown neutral results, while one showed a significant reduction
in heart failure readmissions and a direct total cost reduction of € 3,546 per patient
for 6 months of follow-up.
273
In a Dutch clinical trial, the TEHAF trial, the likelihood of cost-effectiveness for
remote monitoring was 48% (€ 50,000/QALY threshold), probably due to differences between
institutions. One of the most detailed studies was developed for the UK health system
perspective using a Markov model comparing usual treatment, telephone support, or
remote telemonitoring for patients with heart failure after hospital discharge. Assuming
monthly costs of £ 27 for standard care, £ 179 for telephone support, and £ 175 for
telemonitoring during business hours, the most cost-effective strategy was telemonitoring,
with values below £ 20,000 per QALY. The telephone support strategy was very unfavorable,
with an ICER of £ 228,035/QALY compared with telemonitoring.
274
Variables evaluating effectiveness repeat across the studies, notably the reduction
in mortality and in hospitalization frequency. However, accurate cost collection methods,
definition of which cost variables should be collected, and the application of economic
models still lack standard recommendations in the literature. More than 70% of the
studies did not consider at least one category: health care costs, patient and family
expenses, or lost productivity. Many failed to include salaries and benefits, training
time, amortized capital investments, data and follow-up operations, and overhead costs.
Moreover, an important constraint in these economic analyses has been the great heterogeneity
of technology (intervention) and even the control group (alternatives). Technologies
supporting telemedicine services advance at an impressive pace and range from complex
structures and large investments less than 10 years ago to cost-effective solutions
based on cell phones and mobile devices.
256,263
These are distinct services requiring a high initial investment, but most data point
to a return on investment over time due to the volume of patients who then do not
require the use of the traditional health care system.
260,269
Economic assessment methods must, therefore, track the service over time, including
the outcomes of patients receiving care or monitored by the telemedicine services.
Given the diversity of the benefits gained, the applied cost methodology - dedicated
data reflecting conditions of the local health care system - must be evaluated for
a proper understanding of the cost effectiveness of these technologies.
5.4. Economic Evaluations of Telemedicine in Brazil
These new technologies are being gradually introduced in the routine of hospitals,
clinics, and offices in the private and public sectors in Brazil. The first of these
technologies to be applied was the transmission of ECG data for remotely reporting.
In 1994, the company Telecardio started using this technology, transmitting the tests
by telephone, and in 1995, the Instituto do Coração (InCor) created a service called
ECG-FAX. Later, in 2005, a telecardiology system or Minas Telecardio project was implemented
at UFMG’s Clinics Hospital.
275
In 2007, the Ministry of Health, aiming to develop actions to support primary care
teams through permanent education and virtual technologies, created the Telessaúde
Brasil program, later renamed Telessaúde Brasil Redes. Nine Brazilian Telehealth Centers
(Núcleos de Telessaúde no Brasil) were initially established, offering teleconsulting
(consultation between professionals) and telediagnosis (ECG) in the public sector.
Currently, several companies offer remote reporting of ambulatory BP monitoring (ABPM),
Holter, and remote echocardiography and imaging analysis. AI is currently used in
teleconsulting and is at an advanced stage in the preparation of diagnostic test reports.
Telemonitoring of patients with heart failure is also ongoing in Brazil.
The routine use of these technologies in Brazil is an indirect indication of their
effectiveness, and the continuing operation of companies in this sector is an indirect
measure of cost-effectiveness, although there are few formal studies on this subject
in Brazil.
At least two initiatives for the application of new technologies in cardiology in
Brazil focused on these cost-effectiveness studies: 1) the telemedicine service and
remote patient monitoring of InCor at the University of São Paulo Medical School and
2) the telediagnosis and teleconsultation system of the Minas Gerais Teleasssistance
Network (RTMG) of the Clinics Hospital at UFMG.
In addition to the results from telecardiology services, an analysis of savings estimates
for the state of Rio Grande do Sul through the TelessaúdeRS project, which offers
20 clinical specialty teleconsultation services, was conducted and are presented in
this chapter as a third approach to an economic evaluation of telemedicine in Brazil.
5.5. InCor-FMUSP Telemedicine and Patient Monitoring Service
Stevens et al. evaluated the economic burden and impact on patients’ disability of
four main heart conditions - heart failure, AMI, AFib, and hypertension - in 2015
in Brazil.
276
Specifically for hypertension, the authors assessed the cost effectiveness of conventional
care versus telemedicine and structured telephone support over a 30-year time horizon
after 2015. A summary of the results found in the study is shown in Table 5.2.
Table 5.2
Results of comparison: treatment of hypertensive patients with conventional care through
Telemedicine and through a structured telephone support system over a 30-year timeframe
from 2015
Conventional care
Telemedicine
Structured telephone support
Total cost (R$)
5,832
55,930
49,870
Incremental cost (R$)
50,098
44,038
Additional life-years
1
1.89
1.61
Cost per additional life-year (R$/year)
26,437
27,281
1
Additional life-years reflect the impact of longevity on the patient's quality of
life.
Both technologies were considered cost effective by the authors, assuming the standard
defined by the WHO of an intervention being considered cost effective when having
a cost per life-year between one and three times the gross domestic product per capita
per QALY. However, the Brazilian health authorities have not yet defined the country’s
ICER. Thus, safe inferences cannot be made on whether the procedures applied in telemedicine
within the SUS would be cost effective or not within these scenarios. According to
Brazilian law, products, medications, or procedures included in SUS’ protocols must
be evaluated for safety, efficacy, effectiveness, and cost effectiveness. Therefore,
an effective reference is still needed to validate the economic assessment in question.
277
5.6. Telediagnosis and Teleconsultation System of the Minas Gerais Teleassistance
Network (Rede de Teleassistência de Minas Gerais, RTMG) at the Clinics Hospital of
UFMG
The Telehealth Center (Centro de Telessaúde, CTS HC/UFMG) coordinates the RTMG, a
collaborative network established in 2005 by seven public universities in Minas Gerais:
UFMG, Federal University of Uberlândia (UFU), Federal University of Triângulo Mineiro
(UFTM), Federal University of Juiz de Fora (UFJF), Federal University of São João
Del Rei (UFSJ), State University of Montes Claros (Unimontes), and Federal University
of Jequitinhonha and Mucuri Valleys (UFVJM).
Telecare activities include teleconsultation and telediagnosis. Teleconsultations
are mostly asynchronous through regulatory calls in medicine, nursing, dentistry,
physiotherapy, pharmacy, psychology, nutrition, and speech therapy. Telediagnosis
consists of the analysis and reports of ECGs, ABPM, Holter, and retinography, along
with synchronous cardiology teleconsultations to support critical clinical cases.
The service is registered in the CRM of the State of Minas Gerais.
The RTMG activity was initiated with the research project Minas Telecardio in 2006,
implementing a telecardiology service with ECG reports and teleconsultation in 82
municipalities in Minas Gerais. The RTMG activities expanded over time and currently
connects 814 municipalities with more than 1,000 telehealth units in Minas Gerais.
Within the ONTD project of the Ministry of Health, it began to offer nationwide telecardiology
services on September 2017, and currently serves 90 municipalities in the states of
Acre, Bahia, Ceará, Mato Grosso and Roraima. This expansion was partly the result
of studies proving the cost effectiveness of the system for the main RTMG funders
(Ministry of Health and Minas Gerais State Department of Health).
Using the results of the Minas Telecardio Project, Andrade et al.
278
compared the cost-benefit ratio of remote ECG reporting, considering the hypothesis
of economic benefit in performing ECGs in the telecardiology project compared with
the referral of the patient to perform ECG examination at another location.
278
The study was conducted between June 2006 and November 2008 in 82 municipalities in
rural areas of the state of Minas Gerais. Each municipality received a microcomputer
with a digital electrocardiograph machine and had the possibility of forwarding the
ECG recordings and establishing communication with the cardiology department at university
hospitals of the RTMG. The costs of the project were divided into two categories:
related to the implementation and related to the maintenance of the telecardiology
system. The cost of moving patients was assessed, including the cost of transportation
(using city-provided resources), the cost of food during their absence from home,
and the cost of a missed working day (both paid by the patient). The cost-benefit
without inclusion (perspective of the public health service) and with inclusion (perspective
of the society) of the patients’ costs were evaluated. For the face-to-face scenario,
the cost of ECG and cost of the consultation were added (R$ 5.15 and R$ 10.00, respectively,
based on the SUS’ reference table). The sources of data for the analysis were mainly
the National Household Sample Survey (Pesquisa Nacional de Amostra de Domicílios,
PNAD), SIA-SUS Ambulatory Information System, and CNES.
Considering the cost of implementation and maintenance of the project of R$ 1,818,282.87
and the number of examinations performed in the 30-month period (62,865 examinations
from August 2006 to December 2008), the unit cost of each remote report was R$ 28.92.
A summary of the results is shown in Table 5.3.
Table 5.3
Comparison of costs between the alternatives remote report and face-to-face report
in the Minas Telecardio Project
278
Scenario
Cost (R$)
Difference (R$)
1. Remote report
28.92
2. Face-to-face report without costs of patient displacement
30.91
1.99
3. Face-to-face report with costs of patient displacement
49.83
20.91
A sensitivity analysis showed that the results are sensitive to patient travel costs,
particularly related to the driver’s salary and number of patients per vehicle. Given
the small difference between scenarios 1 and 2, it can be concluded that, in some
situations, telecardiology may not be more economical from the point of view of public
health service.
At that time, the system had a relatively low output and, since the activities have
a high fixed cost, resulted in a high cost for the remote report. With the expansion
of the system to other municipalities, the cost of the activities reduced, increasing
the cost effectiveness of the system.
In 2007, the Ministry of Health, with resources from PAHO, engaged the CTS HC/UFMG
in the project “Analysis of the Financial Management of Telehealth Services Applied
to Primary Care.” In the project, the analysis of the economic sustainability of the
application of telehealth in primary care was based on a comparison of costs between
two scenarios:
Face-to-face care: when the patient is treated in primary care and requires to be
subsequently referred to secondary level care;
Remote care: when the primary care physician receives remote support through a telehealth
service, and this support avoids the referral of the patient.
The results refer to 20 municipalities participating in the National Telehealth Project
with reliable data, located in the North/Northeast regions and Jequitinhonha Valley
in Minas Gerais, considered one of the poorest regions of the state. The main results
of the project are shown in table 5.4.
279,280
Table 5.4
Comparison of average costs (R$/month/municipality) between face-to-face and remote
care in the project "Analysis of Financial Management of Telehealth Services Applied
in Primary Care"
279,280
Cost item (R$/month)
Face-to-face care
Remote care
Patients referral
2,399.58
697.78
System implementation
56.03
Equipment depreciation in the municipality
101.97
Equipment maintenance in the municipality
40.79
Capital cost of equipment
51.92
Activities of remote care*
210.80
Total
2,398.58
1,159.29
*
ECG report, case discussion and teleconsultation.
This project presented more realistic results compared with the previous one, as it
collected information about the cost of patient referral directly in the municipalities.
However, it maintained the sample of the participating municipalities concentrated
in the same region of the state, the Jequitinhonha Valley, which has a low human development
index (HDI). In 2009, the Minas Telecardio Expansion project expanded the service
to higher HDI regions for a final sample of 66 other municipalities. The results were
similar to those of the previous project and are shown in table 5.5.
281-283
Table 5.5
"Economic Analysis and Impact of the Application of Telehealth Services in Primary
Care in Municipalities of Minas Gerais"
282
Average cost of each telehealth activity in 2010
R$ 10.68
Average fixed cost of patient referral
R$ 41.77
Savings to the municipality by avoided referral
R$ 71.11
Minimum monthly number of referrals reduction per municipality to enable the system
4.28
Minimum monthly number of activities per municipality to enable the system
5.5
Average monthly number of activities by municipality in 2010
28.5
Investment by SES/MG (2005-2009)
R$ 11,599,638.00
Savings for the health care system (from June 2006 to July 2011)
R$ 31,970,549.13
Return on Investment (ROI) (savings:investment)
2.76:1
% of referrals avoided
78%
Minimum referral distance for system viability (approximate)
54 Km
Average cost of each telehealth activity in 2010*
R$ 10.68
*
ECG report, case discussion and teleconsultation.
Recently, a study (pending publication) was conducted to assess the cost effectiveness
of the ONTD. The ONTD is a project of the Ministry of Health to offer telecardiology
services (ECG reports and teleconsultations) to all Brazilian states, for which the
CTS HC/UFMG was chosen as Specialist Center, that is, the service provider. State
Telehealth Centers receive funding from the Ministry of Health to train and implement
care in municipalities of their state, previously agreed with the State Health Departments,
which may forward 24/7 their test requests and teleconsultations to the Expert Center.
The requests may be elective or urgent. The report is available on a platform, and,
when necessary, local physicians can ask questions as part of teleconsultation. An
alert system informs physicians and nurses about critically emergent situations. The
effectiveness of the system has been proven by performance indicators (time to submit
tests, waiting time for analysis of urgent/elective reports, time to the first analysis
of the report, number of tests requested by the municipality, etc.), and user satisfaction.
The results proved the effectiveness of the system by replacing alternatives previously
available to obtain the test/report (referral of the patient, periodic visits of the
cardiologist to the municipality, and outsourcing of tests to private companies/clinics).
In terms of these alternatives, the cost of the test by the ONTD is about five times
lower, demonstrating cost effectiveness.
5.7. Analysis of the Economic Impact of the TelessaúdeRS Teleconsulting Service
An economic analysis of the TelessaúdeRS service was performed to evaluate the financial
results of the service generated to the state. Teleconsultations are offered for various
specialties, in addition to the contribution to the regulation of waiting lists for
the state of Rio Grande do Sul. Teleconsultations in endocrinology, gastroenterology,
proctology, rheumatology, and urology were selected as samples.
The cost per teleconsultation (R$ 110.29) was evaluated considering data from 8 months
of service and included costs of the physical structure of the service (rent, energy,
depreciation, server capacity, among others) and payment of professionals. As a premise,
all teleconsultations and regulations that resulted in the cancellation of the face-to-face
consultation would represent, for the state, savings in patient transportation to
Porto Alegre (which is variable according to the municipality of origin) and payment
of face-to-face consultation to the municipality (R$ 100.00). Estimated savings were
calculated as the difference between the cost of performing canceled teleconsultations
and the cost of face-to-face care.
The analysis identified that over 21 months (October 2016 to June 2018), the state
saved R$ 2,287,121.78, of which 47% were related to consultations and 53% to transportation
services. For teleconsultation, the service was found to be attractive up to the amount
of payment per consultation of R$ 38.95, considering the average cost of transportation.
The municipalities with the greatest distances from Porto Alegre had higher savings,
except for four municipalities neighboring Porto Alegre, which had a higher number
of avoided teleconsultations than the others’ average.
Of note, a comparison of these results with those of other states and services should
consider that the services of Telessaúde are provided by scholars and “CLT” professionals
(employees following the Brazilian Labor Laws); therefore, Telessaúde can operate
at a lower cost per teleconsultation. The operation of a similar service including
only “CLT” employees would increase the cost per each teleconsultation and require
additional economic analysis.
The WHO considers cost effective those interventions with ICER between 1 and 3 times
the gross domestic product per capita per life-year, unless adjusted for QALY.
6. Recommendations
The Brazilian Society of Cardiology, due to the growing interest in the use of telemedicine
for the expansion of health care, particularly in the area of cardiology, prepared
this guideline to inform the medical category and society in general on the scientific
and technological basis of telemedicine applications considering the current scenario.
Even though there is growing enthusiasm for the democratization of information and
communication technologies, it is important to point out that barriers to implementation
persist across the country and must be addressed. The most significant ones are:
update of laws and regulations applicable by the health authorities and CFM;
provision of minimum telecommunications infrastructure in health care facilities,
especially in remote areas;
cost of technology;
need for qualification and training of human resources;
incorporation of technologies in the SUS’ public policy list and in the List of Procedures
and Events in Health of the National Health Agency.
By bringing to light the discussion about telemedicine applications, in addition to
its media repercussion, we seek to provide scientific and technical support for the
elaboration of health care policies consistent with the use of this technology. In
this sense, we must formally incorporate, after due evaluation by the CONITEC, the
various possibilities available today linked to the respective clinical protocols
and therapeutic guidelines (PCDT). Also, in the context of supplementary health, it
is necessary to include in the List of Procedures and Events in Health of the National
Health Agency those with scientific recognition and authorized for current use in
the country.
As discussed in this guideline, with rare exceptions, there is no provision in the
Brazilian Hierarchical Classification of Medical Procedures (which is a condition
for inclusion in the NHA coverage list) for common procedures in telemedicine. Generic
coding is used, with descriptions that are broad in nature and allow for likelihood
use, such as code 2.01.01.20-1 (clinical and electronic evaluation of a patient with
a cardiac pacemaker or resynchronization defibrillator or defibrillator). However,
the reimbursement of this service will depend on the health care provider’s sole decision.
This fact limits the applicability of telemedicine in the field of supplementary health,
with the related consequences.
In 2015, the Brazilian Society of Cardiology, through the Telecardiology Guideline
for the Care of Patients with Acute Coronary Syndrome and other Heart Diseases,
133
made recommendations on this topic. However, the current version of the broader guideline
addresses new applications for telecardiology, especially those already incorporated
into the health care system. It still deals with future perspectives, such as the
use of telerobotics and AI. The authors, focusing on current scientific evidence and
cost effectiveness, have updated the recommendations to guide public and private health
care providers on judicious use of telemedicine applications in Brazil.
Table 6.1 summarizes the recommendations outlined in this guideline.
Table 6.1
Recommendations for the practice of Telemedicine in Brazil
Clinical indication
Class of indication
Level of evidence
references
Teleconsultation
Teleconsultation assists general practitioners from remote areas in the clinical
evaluation of patients with suspected or established cardiovascular disease, being
cost-effective from the SUS perspective
IIa
B
76,99,100,101,278,279
,
280,281,282
Teleconsultation assists physicians working in emergency care in the management
of cases of acute cardiovascular diseases
IIa
C
133,134,137,138,139,140
,
141,142,143,144,145,149
Teleconsultation assists in regulating access to specialized care in patients
with suspected or established cardiovascular disease
IIa
C
101,102,103,104
Telediagnosis
Tele-electrocardiography is a feasible and effective alternative to offer electrocardiography
in health care systems, and is particularly useful and cost effective in primary care
and remote locations
I
B
61,105,106,107,278
Telemedicine with transmission of electrocardiographic report in pre-hospital
care of patients with suspected acute myocardial infarction reduces cardiovascular
outcomes and early and late mortality
I
B
87,137,138,139,140,141
,
142,143,144,145
,
Tele-echocardiography with teleconsultation is effective in the early detection
of congenital heart disease in newborns
IIa
B
182,183,306
Tele-echocardiography allows the early detection of subclinical cases of rheumatic
heart disease in children and adolescents
IIa
B
111,112,307
Tele-echocardiography in primary care allows early detection of cases of heart
disease and may help prioritize referrals to specialized care.
IIb
C
109,110,112,114
Transmission of tomography and cardiac resonance imaging by telemedicine can be
performed:
- to obtain a second opinion
IIa
C
190,192
- for discussion in "Heart Teams"
IIa
C
190,192
- for remote support in emergency cases
IIa
C
190,192
- in sporadic routine cases
IIa
C
190,192
- for routine transmission of all cardiovascular imaging tests to specialized
centers or groups
IIb
C
190,192
Telemonitoring
Self-monitoring of blood pressure with telemonitoring helps in treatment control
and adherence effective in reducing hospitalizations due to heart failure
IIa
B
75,77,78,127,128,129,130
Noninvasive telemonitoring strategies with structured telephone support are effective
in reducing hospitalizations due to heart failure
I
A
115,116,118,119,120,123
,
124,125,126,158,159,161
Noninvasive telemonitoring strategies with structured telephone support are effective
in reducing mortality in heart failure
IIa
A
115,116,118,119,120,123
,
124,125,126,158,159,161
Remote monitoring of patients with arrhythmias and implantable electrical devices,
in addition to regular telemetric assessments, is effective in reducing outpatient
visits and early detection of device dysfunction.
IIa
B
157,160,162,163,164,165
,
166,167,168,169,170,174
,
175,177,178,179,180
Telerehabilitation of eligible patients with heart failure, with or without left
ventricular dysfunction, with NYHA functional class I-III, is effective in improving
program adherence, quality of life and performance in the 6-minute walking distance
test
IIa
B
148,149,150
151,152
Private communication to send data or ask questions between physicians, in closed
groups of specialists, or among the clinical staff of an institution or chair, safeguarding
professional confidentiality
I
C
191
Private communication between physicians and patients through communication platforms
to send data or ask questions, safeguarding professional confidentiality
IIa
C
191
Related collections
Most cited references205
- Record: found
- Abstract: found
- Article: not found
Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial.

- Record: found
- Abstract: found
- Article: found
Factors Determining the Success and Failure of eHealth Interventions: Systematic Review of the Literature
- Record: found
- Abstract: found
- Article: not found