- Record: found
- Abstract: found
- Article: found
PD-1 IC Inhibition Synergistically Improves Influenza A Virus-Mediated Oncolysis of Metastatic Pulmonary Melanoma

Read this article at
Abstract
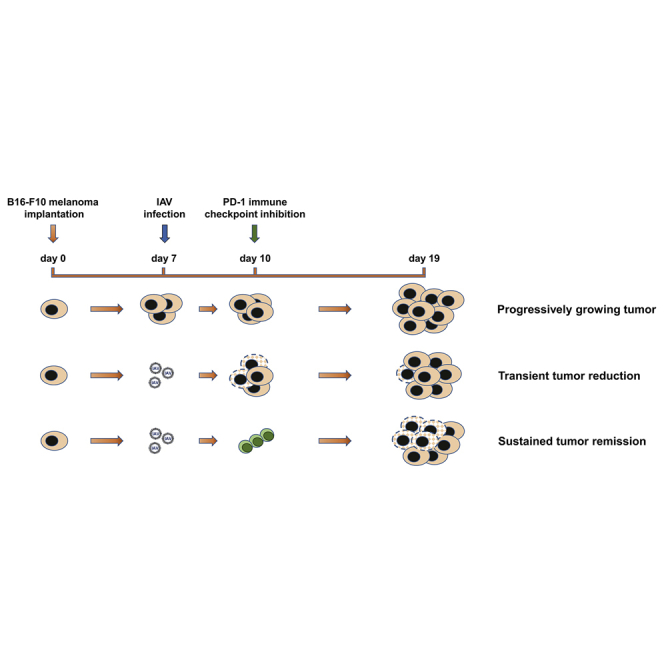
Recently, we showed that infection of primary lung tumor-bearing mice with oncolytic influenza A viruses (IAVs) led to strong virus-induced tumor cell lysis but also to restoration of immune competence of innate immune cells. Murine B16-F10 melanoma cells are known for their high lung tropism and progressive growth. As these cells are also highly permissive for IAVs, we analyzed their oncolytic and immunomodulatory efficiency against pulmonary B16-F10 lung metastases in vivo. IAV infection abrogated the melanoma-mediated immune suppression in the lung and induced a more than 50% cancer cell lysis. The oncolytic effect reached maximal efficacy 3 days post-infection, but it was not sustained over time. In order to maintain the virus-induced anti-tumor effect, mice with melanoma-derived lung cancers were treated in addition to influenza virus infection with an immune checkpoint inhibitor against programmed death-1 receptor (PD-1). The combined IAV and immune checkpoint inhibition (ICI) therapy resulted in a sustained anti-tumor efficacy, keeping the lung melanoma mass at day 12 of IAV infection still reduced by 50% over the control mice. In conclusion, ICI treatment strongly enhanced the oncolytic effect of influenza virus infection, suggesting that combined treatment is a promising approach against metastatic pulmonary melanoma.
Graphical Abstract
Abstract
Sitnik and colleagues demonstrate that pulmonary melanoma metastases are permissive to oncolytic influenza A virus infection, even though oncolytic efficacy was not persistent. However, combined application of influenza viruses and PD-1 immune checkpoint inhibition resulted in sustained metastatic cancer remission and abrogation of the immunosuppressive tumor microenvironment.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade.
- Record: found
- Abstract: not found
- Article: not found
Selection of successive tumour lines for metastasis.
- Record: found
- Abstract: found
- Article: not found