- Record: found
- Abstract: found
- Article: found
Immune Checkpoints as Therapeutic Targets in Autoimmunity
Read this article at
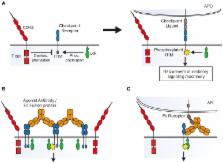
Abstract
Antibodies that block the immune checkpoint receptors PD1 and CTLA4 have revolutionized the treatment of melanoma and several other cancers, but in the process, a new class of drug side effect has emerged—immune related adverse events. The observation that therapeutic blockade of these inhibitory receptors is sufficient to break self-tolerance, highlights their crucial role in the physiological modulation of immune responses. Here, we discuss the rationale for targeting immune checkpoint receptors with agonistic agents in autoimmunity, to restore tolerance when it is lost. We review progress that has been made to date, using Fc-fusion proteins, monoclonal antibodies or other novel constructs to induce immunosuppressive signaling through these pathways. Finally, we explore potential mechanisms by which these receptors trigger and modulate immune cell function, and how understanding these processes might shape the design of more effective therapeutic agents in future.
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans.
- Record: found
- Abstract: found
- Article: not found
Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis.
- Record: found
- Abstract: found
- Article: not found