- Record: found
- Abstract: found
- Article: found
Unlocking NuriPep 1653 From Common Pea Protein: A Potent Antimicrobial Peptide to Tackle a Pan-Drug Resistant Acinetobacter baumannii

Read this article at
Abstract
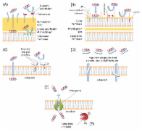
While the antibiotic era has come and gone, antimicrobial peptides (AMPs) hold promise as novel therapies to treat multidrug resistant (MDR) pathogens in an age where the threat of multidrug resistance escalates worldwide. Here, we report the bactericidal properties of NuriPep 1653, a novel 22 mer and non-modified peptide. NuriPep 1653 was identified within the sequence of the non-antimicrobial P54 protein, which is involved in nutrient reservoir activity in Pisum sativum. Total bacterial clearance of Acinetobacter baumannii cells (1 × 10 8 cells/mL) was observed using only 4 × MIC (48 μg/mL) of NuriPep 1653 after just 20 min of treatment. We uncovered a synergistic interaction between NuriPep 1653 and another antimicrobial peptide, colistin. The MIC of NuriPep 1653 and colistin dropped from 12 and 8 μg/mL to 2 and 1 μg/mL, respectively, when they were combined. NuriPep 1653 exhibits no cytotoxicity in different human cell lines and has a low propensity to induce bacterial resistance in a colistin resistant clinical isolate of A. baumannii. The existence of these peptides embedded in proteins unearths potentially new classes of antimicrobials with activity against clinically relevant pathogens. Our findings push the boundaries of traditional peptide discovery and represent a leading edge for natural bioactive compounds which may have a common existence in nature but remain unexposed.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Mechanisms of antimicrobial peptide action and resistance.
- Record: found
- Abstract: found
- Article: not found