- Record: found
- Abstract: found
- Article: found
Virus-like particle display of HER2 induces potent anti-cancer responses
Read this article at
ABSTRACT
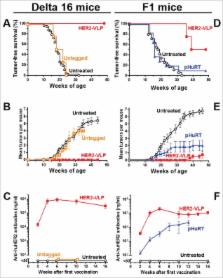
Overexpression of human epidermal growth factor receptor-2 (HER2) occurs in 20–30% of invasive breast cancers. Monoclonal antibody therapy is effective in treating HER2-driven mammary carcinomas, but its utility is limited by high costs, side effects and development of resistance. Active vaccination may represent a safer, more effective and cheaper alternative, although the induction of strong and durable autoantibody responses is hampered by immune-tolerogenic mechanisms. Using a novel virus-like particle (VLP) based vaccine platform we show that directional, high-density display of human HER2 on the surface of VLPs, allows induction of therapeutically potent anti-HER2 autoantibody responses. Prophylactic vaccination reduced spontaneous development of mammary carcinomas by 50%-100% in human HER2 transgenic mice and inhibited the growth of HER2-positive tumors implanted in wild-type mice. The HER2-VLP vaccine shows promise as a new cost-effective modality for prevention and treatment of HER2-positive cancer. The VLP platform may represent an effective tool for development of vaccines against other non-communicable diseases.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: not found
Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2.
- Record: found
- Abstract: found
- Article: not found
Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene
- Record: found
- Abstract: found
- Article: not found