- Record: found
- Abstract: found
- Article: found
An improved mosquito electrocuting trap that safely reproduces epidemiologically relevant metrics of mosquito human-feeding behaviours as determined by human landing catch

Read this article at
Abstract
Background
Reliable quantification of mosquito host—seeking behaviours is required to determine the efficacy of vector control methods. For malaria, the gold standard approach remains the risky human landing catch (HLC). Here compare the performance of an improved prototype of the mosquito electrocuting grid trap (MET) as a safer alternative with HLC for measuring malaria vector behaviour in Dar es Salaam, Tanzania.
Methods
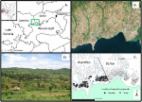
Mosquito trapping was conducted at three sites within Dar es Salaam representing a range of urbanicity over a 7-month period (December 2012–July 2013, 168 sampling nights). At each site, sampling was conducted in a block of four houses, with two houses being allocated to HLC and the other to MET on each night of study. Sampling was conducted both indoors and outdoors (from 19:00 to 06:00 each night) at all houses, with trapping method (HLC and MET) being exchanged between pairs of houses at each site using a crossover design.
Results
The MET caught significantly more Anopheles gambiae sensu lato than the HLC, both indoors (RR [95 % confidence interval (CI)]) = 1.47 [1.23–1.76], P < 0.0001 and outdoors = 1.38 [1.14–1.67], P < 0.0001). The sensitivity of MET compared with HLC did not detectably change over the course of night for either An. gambiae s.l. (OR [CI]) = 1.01 [0.94–1.02], P = 0.27) or Culex spp. (OR [CI]) = 0.99 [0.99–1.0], P = 0.17) indoors and declined only slightly outdoors: An. gambiae s.l. (OR [CI]) = 0.92 [0.86–0.99], P = 0.04), and Culex spp. (OR [CI]) = 0.99 [0.98–0.99], P = 0.03). MET-based estimates of the proportions of mosquitoes caught indoors ( P i ) or during sleeping hours ( P fl ), as well as the proportion of human exposure to bites that would otherwise occurs indoors (π i ), were statistically indistinguishable from those based on HLC for An. gambiae s.l. (P = 0.43, 0.07 and 0.48, respectively) and Culex spp. (P = 0.76, 0.24 and 0.55, respectively).
Related collections
Most cited references102
- Record: found
- Abstract: found
- Article: not found
Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction.

- Record: found
- Abstract: found
- Article: found
Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania
- Record: found
- Abstract: found
- Article: found