- Record: found
- Abstract: found
- Article: found
Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells ☆
Read this article at
Abstract
Neurons rely on the release and subsequent cleavage of GSH to cysteinylglycine (CysGly) by astrocytes in order to maintain optimal intracellular GSH levels. In neurodegenerative diseases characterised by oxidative stress, neurons need an optimal GSH supply to defend themselves against free radicals released from activated microglia and astroglia. The rate of GSH synthesis is controlled largely by the activity of γ-glutamyl cysteine ligase. Expression of γ-glutamyl cysteine ligase and of the Xc- system, which facilitates cystine uptake, is regulated by the redox-sensitive transcription factor, nuclear factor erythroid-2-related factor 2 (Nrf2). Compounds that can activate the Nrf2-ARE pathway, referred to as ‘Nrf2 activators’ are receiving growing attention due to their potential as GSH-boosting drugs.
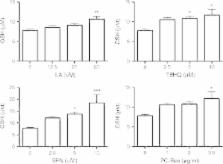
This study compares four known Nrf2 activators, R-α-Lipoic acid (LA), tert-butylhydroquinone (TBHQ), sulforaphane (SFN) and Polygonum cuspidatum extract containing 50% resveratrol (PC-Res) for their effects on astroglial release of GSH and CysGly. GSH levels increased dose-dependently in response to all four drugs. Sulforaphane produced the most potent effect, increasing GSH by up to 2.4-fold. PC-Res increased GSH up to 1.6-fold, followed by TBHQ (1.5-fold) and LA (1.4-fold). GSH is processed by the ectoenzyme, γ-glutamyl transpeptidase, to form CysGly. Once again, SFN produced the most potent effect, increasing CysGly by up to 1.7-fold, compared to control cells. TBHQ and PC-Res both induced fold increases of 1.3, followed by LA with a fold increase of 1.2. The results from the present study showed that sulforaphane, followed by lipoic acid, resveratrol and Polygonum multiflorum were all identified as potent “GSH and Cys-Gly boosters”.
Graphical abstract
Highlights
-
•
R-α-Lipoic acid (LA), tert-butylhydroquinone (TBHQ), sulforaphane (SFN) and Polygonum cuspidatum extract containing 50% resveratrol (PC-Res) increase astroglial release of GSH.
-
•
Sulforaphane produced the most potent effect, increasing GSH by up to 2.4-fold. PC-Res increased GSH up to 1.6-fold, followed by TBHQ (1.5-fold) and LA (1.4-fold). GSH is processed by the ectoenzyme, γ-glutamyl transpeptidase, to form CysGly.
-
•
Once again, SFN produced the most potent effect, increasing CysGly by up to 1.7-fold, compared to control cells. TBHQ and PC-Res both induced fold increases of 1.3, followed by LA with a fold increase of 1.2.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress.
- Record: found
- Abstract: found
- Article: not found
Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor.
- Record: found
- Abstract: found
- Article: not found
