- Record: found
- Abstract: found
- Article: found
Anti-high mobility group box-1 monoclonal antibody treatment provides protection against influenza A virus (H1N1)-induced pneumonia in mice
Read this article at
Abstract
Introduction
Provision for the emergence of an influenza pandemic is an urgent issue. The discovery of a novel anti-influenza therapeutic approach would increase the effectiveness of traditional virus-based strategies. This study was undertaken to evaluate the therapeutic effects of anti-high mobility group box-1 (HMGB1) monoclonal antibody (mAb) treatment on influenza A virus (H1N1)-induced pneumonia in mice.
Methods
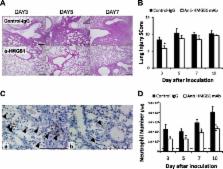
Nine-week-old male C57BL/6 mice were inoculated with H1N1, then anti-HMGB1 mAb or control mAb were administered intravenously at 1, 24 and 48 hours after H1N1 inoculation and the survival rate was analyzed. Lung lavage and histopathological analysis were performed on days 3, 5, 7 and 10 after inoculation.
Results
Anti-HMGB1 mAb significantly improved the survival rate of H1N1-inoculated mice (1 out of 15 versus 8 out of 15 deaths in the anti-HMGB1 mAb-treated group versus the control mAb-treated group, p < 0.01), although the treatment did not affect virus propagation in the lungs. The treatment also significantly attenuated histological changes and neutrophil infiltration in the lungs of H1N1-inoculated mice. This was associated with inhibition of HMGB1 and suppression of inflammatory cytokine/chemokine expression and oxidative stress enhancement, which were observed in H1N1-inoculated mice. The expression of receptor for advanced glycation end products and nuclear factor κB was attenuated by the treatment.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
HMG-1 as a late mediator of endotoxin lethality in mice.
- Record: found
- Abstract: found
- Article: not found
Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico.
- Record: found
- Abstract: found
- Article: not found