- Record: found
- Abstract: found
- Article: found
Skeletal Muscle Mass Reduction Velocity as a Simple Prognostic Indicator for Patients with Metastatic Urothelial Carcinoma Receiving Second-Line Chemotherapy
Read this article at
Abstract
Background:
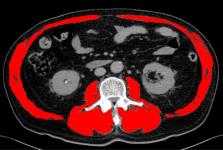
Patients with metastatic urothelial carcinoma (mUC) have an uncertain prognosis. The aim of the current study was to evaluate the prognostic potential of a skeletal muscle mass reduction index measured by computed tomography (CT) for mUC patients undergoing second-line gemcitabine and docetaxel (GD) chemotherapy.
Methods:
We retrospectively reviewed 44 patients with mUC who received second-line GD chemotherapy between 2006 and 2015 in our hospital. Skeletal muscle area (SMA) at the third lumbar vertebra was measured using CT images obtained from medical records, and a skeletal muscle index (SMI) was calculated for each patient as: SMI = SMA / height2. Changes in SMI across timepoints (SMI inclination) were calculated as: SMI inclination = [( SMI/SMI)/duration of the interval between imaging visits]. Patients were then divided into two groups: a “steep” group (SMI inclination < -0.01) and a “gentle” group (SMI inclination ≥ -0.01). Kaplan-Meier curves and multivariate Cox proportional hazards regression models were used to evaluate the relationship between SMI inclination and overall survival (OS).
Results:
There were no differences in patient characteristics between the two groups with respect to median age, gender, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), disease control rate or first-line treatment regimen. OS from the start of second-line GD therapy group was significantly shorter in the “steep” group relative to the “gentle” group. The multivariate analysis revealed that “steep” SMI inclination and presence of anemia were strong predictors of poor prognosis.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1).
- Record: found
- Abstract: found
- Article: not found
Sarcopenia: European consensus on definition and diagnosis
- Record: found
- Abstract: found
- Article: not found