- Record: found
- Abstract: found
- Article: found
Iron in Neurodegeneration – Cause or Consequence?

Read this article at
Abstract
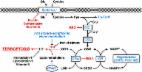
Iron dyshomeostasis can cause neuronal damage to iron-sensitive brain regions. Neurodegeneration with brain iron accumulation reflects a group of disorders caused by iron overload in the basal ganglia. High iron levels and iron related pathogenic triggers have also been implicated in sporadic neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple system atrophy (MSA). Iron-induced dyshomeostasis within vulnerable brain regions is still insufficiently understood. Here, we summarize the modes of action by which iron might act as primary or secondary disease trigger in neurodegenerative disorders. In addition, available treatment options targeting brain iron dysregulation and the use of iron as biomarker in prodromal stages are critically discussed to address the question of cause or consequence.
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: found
Targeting Nrf2 to Suppress Ferroptosis and Mitochondrial Dysfunction in Neurodegeneration
- Record: found
- Abstract: found
- Article: not found