- Record: found
- Abstract: found
- Article: found
Association Between Circulating Ketone Bodies and Worse Outcomes in Hemodialysis Patients
Read this article at
Abstract
Background
Cardiovascular disease is the leading cause of morbidity and mortality in patients receiving hemodialysis. Systemic metabolic perturbation is one of the hallmark abnormalities in patients at high cardiovascular risk. We sought to determine the relationship between circulating ketone body and clinical outcomes in patients with prevalent hemodialysis.
Methods and Results
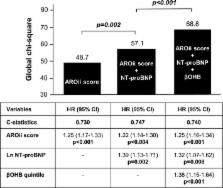
We retrospectively assessed the relationship between serum β‐hydroxybutyrate (β OHB), the most abundant ketone body in the circulation, and prognosis in 405 stable hemodialysis patients. During a mean follow‐up of 3.2±0.9 years, there were 54 major adverse cardiovascular events (defined as cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization attributed to heart failure) and 67 all‐cause deaths. Major adverse cardiovascular events rates increased from 11.1 per 1000 person‐years in the lowest β OHB quintile (<89 μmol/L) to 80.1 per 1000 person‐years in the highest quintile (>409 μmol/L). After adjusting for demographic characteristics, coronary artery disease, and atrial fibrillation, the highest β OHB quintile was associated with increased risk of major adverse cardiovascular events compared with the lowest quintile (hazard ratio, 10.2; 95% confidence interval [3.35–44.0]; P<0.001). Increased quintiles of β OHB were independently and incrementally associated with major adverse cardiovascular events over the model based on an established risk score (the second Analyzing Data, Recognizing Excellence and Optimizing Outcomes cohort score) and N‐terminal pro‐B‐type natriuretic peptide (chi square 39.9 versus 21.7; P<0.001; c‐statistics, 0.713). Sensitivity analyses also confirmed the robustness of association between β OHB and all‐cause death.
Related collections
Most cited references12
- Record: found
- Abstract: found
- Article: not found
Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients.
- Record: found
- Abstract: found
- Article: not found
Phagocyte-derived catecholamines enhance acute inflammatory injury.
- Record: found
- Abstract: found
- Article: not found