- Record: found
- Abstract: found
- Article: not found
Dissolving Polymer Microneedle Patches for Influenza Vaccination
Abstract
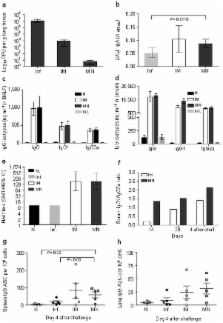
Influenza prophylaxis would benefit from a vaccination method enabling simplified logistics and improved immunogenicity without the dangers posed by hypodermic needles. Here, we introduce dissolving microneedle patches for influenza vaccination using a simple patch-based system that targets delivery to skin’s antigen-presenting cells. Microneedles were fabricated using a biocompatible polymer encapsulating inactivated influenza virus vaccine for insertion and dissolution in the skin within minutes. Microneedle vaccination generated robust antibody and cellular immune responses in mice that provided complete protection against lethal challenge. Compared to conventional intramuscular injection, microneedle vaccination resulted in more efficient lung virus clearance and enhanced cellular recall responses after challenge. These results suggest that dissolving microneedle patches can provide a novel technology for simpler and safer vaccination with improved immunogenicity that could facilitate increased vaccination coverage.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Transdermal drug delivery.
- Record: found
- Abstract: found
- Article: not found
Dissolving microneedles for transdermal drug delivery.
- Record: found
- Abstract: found
- Article: not found
