- Record: found
- Abstract: found
- Article: found
MGP Promotes Colon Cancer Proliferation by Activating the NF-κB Pathway through Upregulation of the Calcium Signaling Pathway

Read this article at
Abstract
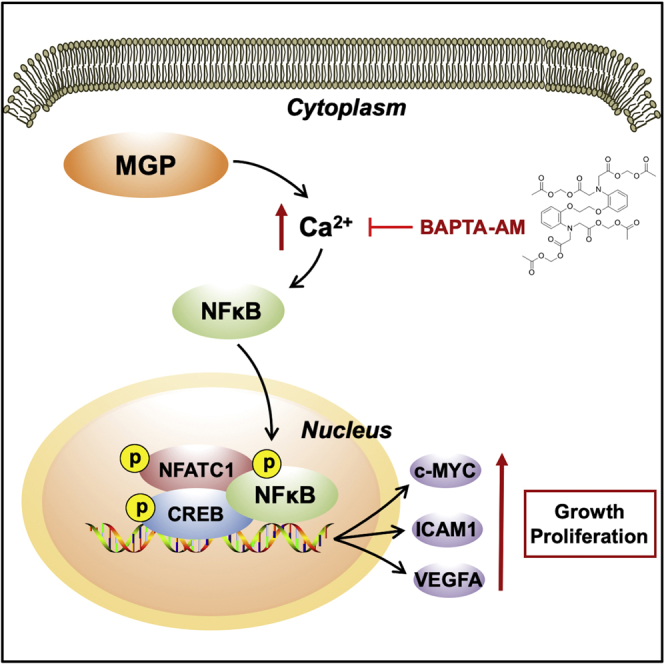
Matrix Gla protein (MGP), an extracellular matrix protein, is mainly associated with the inhibition of calcification in skeleton, coronary artery, and kidney, and more recently it has also been implicated in cancer. However, the biological function of MGP inside cancer cells and its role in colon cancer (CC) remain largely unknown. MGP expression and its association with clinicopathologic characteristics in CC were analyzed by immunohistochemistry and verified by Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) datasets. The effects of MGP on CC cell proliferation were evaluated via knockdown and overexpression experiments in vitro. Mechanisms of MGP in CC were explored by western blots, quantitative real-time PCR, Fluo-3 AM staining, Rhod-2 AM staining, immunofluorescence, and other techniques. Our study confirmed that MGP was upregulated in different stages of CC and associated with a worse prognosis. MGP could enrich intracellular free Ca 2+ concentration and promote nuclear factor κB (NF-κB)/p65 phosphorylation, activating the expression of c-MYC, ICAM-1, and VEGFA. Furthermore, the reduction of intracellular free Ca 2+ concentration and the subsequent growth inhibition effect on CC cells induced by small interfering RNA targeting MGP (siMGP) could be rescued by a higher calcium concentration environment. Therefore, MGP promotes the growth and proliferation of CC cells by enriching intracellular calcium concentration and activating the NF-κB pathway, and it could serve as a potential prognostic biomarker in CC patients.
Graphical Abstract
Abstract
Zhang and colleagues showed that a high expression of matrix Gla protein (MGP) was correlated with colon cancer (CC) progression. They found that MGP enriched intracellular free Ca 2+ concentration and then activated the NF-κB pathway. MGP could act as an oncogene and serve as a diagnostic and prognostic biomarker in CC patients.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found