- Record: found
- Abstract: found
- Article: found
Mycobacterium tuberculosis PPD-induced immune biomarkers measurable in vitro following BCG vaccination of UK adolescents by multiplex bead array and intracellular cytokine staining
Read this article at
Abstract
Background
The vaccine efficacy reported following Mycobacterium bovis Bacillus Calmette Guerin (BCG) administration to UK adolescents is 77% and defining the cellular immune response in this group can inform us as to the nature of effective immunity against tuberculosis. The aim of this study was to identify which cytokines and lymphocyte populations characterise the peripheral blood cellular immune response following BCG vaccination.
Results
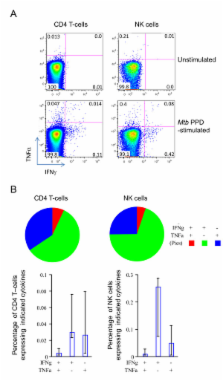
Diluted blood from before and after vaccination was stimulated with Mycobacterium tuberculosis purified protein derivative for 6 days, after which soluble biomarkers in supernatants were assayed by multiplex bead array. Ten out of twenty biomarkers measured were significantly increased (p < 0.0025) 1 month after BCG vaccination when compared to paired samples (n = 12) taken prior to vaccination (IFNγ, TNFα, IL-1α, IL-2, IL-6, IL-10, IL-17, GM-CSF, MIP1α, IP-10). All of these remained detectable by multiplex bead array in samples taken 12 months after BCG vaccination of a partially overlapping adolescent group (n = 12). Intracellular cytokine staining after 24 hour Mycobacterium tuberculosis purified protein derivative stimulation of PBMC samples from the 12 month group revealed that IFNγ expression was detectable in CD4 and CD8 T-cells and natural killer cells. Polyfunctional flow cytometry analysis demonstrated that cells expressing IFNγ alone formed the majority in each subpopulation of cells. Only in CD4 T-cells and NK cells were there a notable proportion of responding cells of a different phenotype and these were single positive, TNFα producers. No significant expression of the cytokines IL-2, IL-17 or IL-10 was seen in any population of cells.
Conclusions
The broad array of biomarker responses detected by multiplex bead array suggests that BCG vaccination is capable, in this setting, of inducing a complex immune phenotype. Although polyfunctional T-cells have been proposed to play a role in protective immunity, they were not present in vaccinated adolescents who, based on earlier epidemiological studies, should have developed protection against pulmonary tuberculosis. This may be due to the later sampling time point available for testing or on the kinetics of the assays used.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge.
- Record: found
- Abstract: found
- Article: not found
Disseminated tuberculosis in interferon gamma gene-disrupted mice
- Record: found
- Abstract: found
- Article: not found