- Record: found
- Abstract: found
- Article: found
Modulation of Immune-Inflammatory Responses in Abdominal Aortic Aneurysm: Emerging Molecular Targets
Read this article at
Abstract
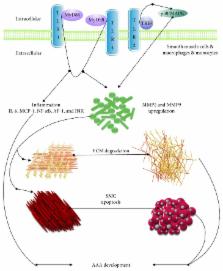
Abdominal aortic aneurysm (AAA), a deadly vascular disease in human, is a chronic degenerative process of the abdominal aorta. In this process, inflammatory responses and immune system work efficiently by inflammatory cell attraction, proinflammatory factor secretion and subsequently MMP upregulation. Previous studies have demonstrated various inflammatory cell types in AAA of human and animals. The majority of cells, such as macrophages, CD4+ T cells, and B cells, play an important role in the diseased aortic wall through phenotypic modulation. Furthermore, immunoglobulins also greatly affect the functions and differentiation of immune cells in AAA. Recent evidence suggests that innate immune system, especially Toll-like receptors, chemokine receptors, and complements are involved in the progression of AAAs. We discussed the innate immune system, inflammatory cells, immunoglobulins, immune-mediated mechanisms, and key cytokines in the pathogenesis of AAA and particularly emphasis on a further trend and application of these interventions. This current understanding may offer new insights into the role of inflammation and immune response in AAA.
Related collections
Most cited references167
- Record: found
- Abstract: found
- Article: not found
Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice.
- Record: found
- Abstract: found
- Article: not found
CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages.
- Record: found
- Abstract: found
- Article: not found