- Record: found
- Abstract: found
- Article: found
Development of antitumor biguanides targeting energy metabolism and stress responses in the tumor microenvironment
Read this article at
Abstract
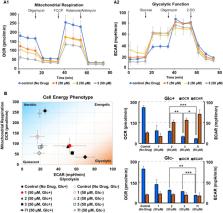
To develop antitumor drugs capable of targeting energy metabolism in the tumor microenvironment, we produced a series of potent new biguanide derivatives via structural modification of the arylbiguanide scaffold. We then conducted biological screening using hypoxia inducible factor (HIF)-1- and unfolded protein response (UPR)-dependent reporter assays and selective cytotoxicity assay under low glucose conditions. Homologation studies of aryl-(CH 2) n-biguanides (n = 0–6) yielded highly potent derivatives with an appropriate alkylene linker length (n = 5, 6). The o-chlorophenyl derivative 7l (n = 5) indicated the most potent inhibitory effects on HIF-1- and UPR-mediated transcriptional activation (IC 50; 1.0 ± 0.1 μM, 7.5 ± 0.1 μM, respectively) and exhibited selective cytotoxicity toward HT29 cells under low glucose condition (IC 50; 1.9 ± 0.1 μM). Additionally, the protein expression of HIF-1α induced by hypoxia and of GRP78 and GRP94 induced by glucose starvation was markedly suppressed by the biguanides, thereby inhibiting angiogenesis. Metabolic flux and fluorescence-activated cell sorting analyses of tumor cells revealed that the biguanides strongly inhibited oxidative phosphorylation and activated compensative glycolysis in the presence of glucose, whereas both were strongly suppressed in the absence of glucose, resulting in cellular energy depletion and apoptosis. These findings suggest that the pleiotropic effects of these biguanides may contribute to more selective and effective killing of cancer cells due to the suppression of various stress adaptation systems in the tumor microenvironment.
Related collections
Most cited references52

- Record: found
- Abstract: found
- Article: not found
Cancer cell metabolism: Warburg and beyond.
- Record: found
- Abstract: found
- Article: not found