- Record: found
- Abstract: found
- Article: found
Postnatal Testicular Activity in Healthy Boys and Boys With Cryptorchidism
Read this article at
Abstract
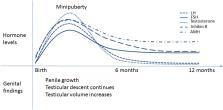
Cryptorchidism, or undescended testis, is a well-known risk factor for testicular cancer and impaired semen quality in adulthood, conditions which have their origins in early fetal and postnatal life. In human pregnancy, the interplay of testicular and placental hormones as well as local regulatory factors and control by the hypothalamic-pituitary (HP) axis, lead to testicular descent by term. The normal masculine development may be disrupted by environmental factors or genetic defects and result in undescended testes. Minipuberty refers to the postnatal re-activation of the HP-testicular (T) axis after birth. During the first weeks of life, gonadotropin levels increase, followed by activation and proliferation of testicular Leydig, Sertoli and germ cells. Consequent rise in testosterone levels results in penile growth during the first months of life. Testicular size increases and testicular descent continues until three to five months of age. Insufficient HPT axis activation (e.g., hypogonadotropic hypogonadism) is often associated with undescended testis and therefore minipuberty is considered an important phase in the normal male reproductive development. Minipuberty provides a unique window of opportunity for the early evaluation of HPT axis function during early infancy. For cryptorchid boys, hormonal evaluation during minipuberty may give a hint of the underlying etiology and aid in the evaluation of the later risk of HPT axis dysfunction and impaired fertility. The aim of this review is to summarize the current knowledge of the role of minipuberty in testicular development and descent.
Related collections
Most cited references114
- Record: found
- Abstract: found
- Article: not found
Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism.
- Record: found
- Abstract: found
- Article: not found
Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B.
- Record: found
- Abstract: found
- Article: not found