- Record: found
- Abstract: found
- Article: found
Repurposed Therapeutic Agents Targeting the Ebola Virus: A Systematic Review
Read this article at
Abstract
Background
The Ebola virus has been responsible for numerous outbreaks since the 1970s, with the most recent outbreak taking place between 2014 and 2016 and causing an international public health emergency. Ebola virus disease (EVD) has a high mortality rate and no approved targeted treatment exists to date. A number of established drugs are being considered as potential therapeutic agents for the treatment of EVD.
Objective
We aimed to identify potential drug repositioning candidates and to assess the scientific evidence available on their efficacy.
Methods
We conducted a systematic literature search in MEDLINE, Embase, and other relevant trial registry platforms for studies published between January 1976 and January 2017. We included drug screening, preclinical studies, and clinical studies on repurposed drugs for the treatment of EVD. The risk of bias for animal studies and nonrandomized clinical studies was assessed. The quality of reporting for case series and case reports was evaluated. Finally, we selected drugs approved by established regulatory authorities, which have positive in vitro study outcomes and at least one additional animal or clinical trial.
Results
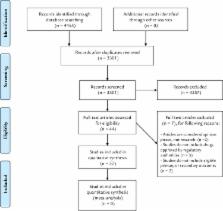
We identified 3301 publications, of which 37 studies fulfilled our inclusion criteria. Studies were highly heterogeneous in terms of study type, methodology, and intervention. The risk of bias was high for 13 out of 14 animal studies. We selected 11 drugs with potential anti-EVD therapeutic effects and summarized their evidence.
Conclusions
Several established drugs may have therapeutic effects on EVD, but the quality and quantity of current scientific evidence is lacking. This review highlights the need for well-designed and conducted preclinical and clinical research to establish the efficacy of potential repurposed drugs against EVD.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: found
SYRCLE’s risk of bias tool for animal studies

- Record: found
- Abstract: found
- Article: found
Are animal models predictive for humans?
- Record: found
- Abstract: found
- Article: not found