- Record: found
- Abstract: found
- Article: found
Tuberculosis Susceptibility and Vaccine Protection Are Independently Controlled by Host Genotype
Read this article at
ABSTRACT
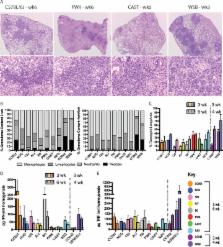
The outcome of Mycobacterium tuberculosis infection and the immunological response to the bacillus Calmette-Guerin (BCG) vaccine are highly variable in humans. Deciphering the relative importance of host genetics, environment, and vaccine preparation for the efficacy of BCG has proven difficult in natural populations. We developed a model system that captures the breadth of immunological responses observed in outbred individual mice, which can be used to understand the contribution of host genetics to vaccine efficacy. This system employs a panel of highly diverse inbred mouse strains, consisting of the founders and recombinant progeny of the “Collaborative Cross” project. Unlike natural populations, the structure of this panel allows the serial evaluation of genetically identical individuals and the quantification of genotype-specific effects of interventions such as vaccination. When analyzed in the aggregate, our panel resembled natural populations in several important respects: the animals displayed a broad range of susceptibility to M. tuberculosis, differed in their immunological responses to infection, and were not durably protected by BCG vaccination. However, when analyzed at the genotype level, we found that these phenotypic differences were heritable. M. tuberculosis susceptibility varied between lines, from extreme sensitivity to progressive M. tuberculosis clearance. Similarly, only a minority of the genotypes was protected by vaccination. The efficacy of BCG was genetically separable from susceptibility to M. tuberculosis, and the lack of efficacy in the aggregate analysis was driven by nonresponsive lines that mounted a qualitatively distinct response to infection. These observations support an important role for host genetic diversity in determining BCG efficacy and provide a new resource to rationally develop more broadly efficacious vaccines.
IMPORTANCE
Tuberculosis (TB) remains an urgent global health crisis, and the efficacy of the currently used TB vaccine, M. bovis BCG, is highly variable. The design of more broadly efficacious vaccines depends on understanding the factors that limit the protection imparted by BCG. While these complex factors are difficult to disentangle in natural populations, we used a model population of mice to understand the role of host genetic composition in BCG efficacy. We found that the ability of BCG to protect mice with different genotypes was remarkably variable. The efficacy of BCG did not depend on the intrinsic susceptibility of the animal but, instead, correlated with qualitative differences in the immune responses to the pathogen. These studies suggest that host genetic polymorphism is a critical determinant of vaccine efficacy and provide a model system to develop interventions that will be useful in genetically diverse populations.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Disseminated tuberculosis in interferon gamma gene-disrupted mice
- Record: found
- Abstract: found
- Article: not found
Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature.
- Record: found
- Abstract: found
- Article: not found