- Record: found
- Abstract: found
- Article: found
Genetics and genomic medicine in Saudi Arabia
research-article
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Saudi Arabia: The New Arabia
Saudi Arabia (officially, Kingdom of Saudi Arabia) was established as a sovereign
country in 1932, by its founder King Abdulaziz Al-Saud who led a successful campaign
that lasted 30 years following the conquest of Riyadh (official capital) to unite
large swathes of the Arabian Peninsula. With an area of 2,150,000 km2, Saudi Arabia
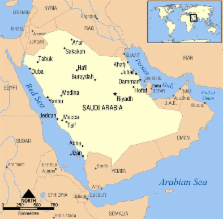
occupies 80% of historical Arabia and is the 13th largest country in the world (Fig.
1). The King in Saudi Arabia also presides over the Council of Ministers, which represents
the executive branch of Government although it also has the power to approve and veto
legislations proposed by the Shura Council (Parliament), which has a largely advisory
role.
Figure 1
Map of Saudi Arabia (Source: Wikimedia).
The presence of the Islamic holiest sites in Mecca and Medina has endowed Saudi Arabia
with a special status to the world's one billion Muslims since its establishment (the
King of Saudi Arabia carries the official title of The Custodian of The Two Holy Mosques).
With the discovery of massive oil reserves and other natural resources shortly after
it was founded, Saudi Arabia has enjoyed vast revenues that made it possible to implement
a very ambitious social and economic modernization strategy that transformed a mostly
illiterate population to one with a literacy rate of 94.4% (98.1% for those <50 years
of age who represent 75% of the population), and to become a member of G20, a forum
of the world's 20 major economies.
Population Structure
The population size in Saudi Arabia as of 2012, is 29.2 million, mostly Saudi nationals
but with a significant minority (∼30%) of expatriates who come from many countries
around the world, for example, there are >2.8 million Indians and around 1 million
Pilipino. In addition to the ethnic Arabs who represent the overwhelming majority,
there is a significant minority of Saudi nationals who largely descended from waves
of immigrants who opted to stay in close proximity to the Holy Mosques in Mecca and
Medina and were assimilated after the founding of modern Saudi Arabia. This minority
represents a remarkably diverse group of ethnicities mostly from Asia and Africa.
Virtually all Saudi nationals proclaim Islam as their religious affiliation. This
has important implications when I discuss the ethical aspects surrounding genetics
and genomic medicine in the country.
The relatively large size of Saudi families (average 6) has its roots in ancient Arabia
when the large number of children was a source of pride. Large family size was further
encouraged by Islam, the faith that was quickly embraced by nearly all inhabitants
of Arabia shortly after its introduction >1400 years ago. Consanguinity is also an
ancient practice that continues to be observed in more than half of the contemporary
marriages in Saudi Arabia. Islam regulated this practice by proscribing a strict code
that absolutely prohibits marriage between first and second degree relatives, but
permits marriage between cousins. This permission is not equivalent to encouragement
as some erroneously infer; Prophet Mohammed himself did marry women who were unrelated
to him including one from a Jewish tribe. In addition to the commonly cited factor
of “wealth preservation”, a powerful and yet less known mechanism that perpetuates
the practice of consanguinity is the traditional view that marriage is the natural
course of women such that families should arrange within themselves to leave no woman
unmarried. Although there are no more recent published data, the rates from 2008 suggest
no decline compared to the rates published two or more decades earlier, indicating
the resistance of this social practice to the wave of modernization that has swept
the country over that period of time (El-Mouzan et al. 2007; Warsy et al. 2014). It
remains to be seen if the recently published declining rates of consanguinity in neighboring
countries with very similar cultural norms will replicate in Saudi Arabia. What is
obvious, however, is that consanguinity will remain a powerful factor in shaping the
landscape of genetic disorders in Saudi Arabia for the foreseeable future.
Health Services in Saudi Arabia
Saudis in general favor a strong contribution of Government to their life in return
for its control over the country's vast natural resources. Just like education, which
is offered freely from K12 to doctorate and postdoctorate degrees, health is also
provided freely for all citizens. Expatriates are entitled to health insurance provided
by their employers as mandated by law and receive their health care through an extensive
network of private-run healthcare systems. The public healthcare system is mostly
under the governance of the Ministry of Health (MOH) and consists of 2259 primary
care centers, and 259 hospitals. The doctor/population ratio and hospital/population
ratios at 24.4/10,000 and 20.7/10,000, respectively, are below that of many developed
countries but newer plans have been revealed to improve this ratio. Law-enforcement
personnel are entitled, in addition to MOH-run health care, to a large network of
primary care centers and hospitals that are run by the Ministry of Interior. Similarly,
military and National Guard personnel and their families enjoy the additional medical
services that are administered by the respective agencies. The author's own institution
(KFSHRC) is a general organization that is funded by the Government and offers highly
specialized health care independent of MOH. The private sector consists of a vast
network of private practices, usually in the form of polyclinics that fall under one
administration, as well as secondary and tertiary hospitals. Although this sector
represents the sole healthcare provider for noncitizens, many citizens also receive
their healthcare in the private sector by choice, for example, to avoid a long wait-time
in the public sector. This fragmentation of healthcare delivery has created a number
of challenges towards the adoption of a national healthcare strategy equivalent to
other countries with socialized medicine, for example, NHS in the UK. Mortality rate
statistics are well below the global average but not yet on par with those of more
developed countries. For example, mortality rate of children less than 5 is 12/1000
and maternal mortality is 7/100,000 births (global average is 44/1000 and 209.1/100,000,
and Western Europe has an average of 3.9/1000 and 6.3/100,000, respectively) (Kassebaum
et al. 2014; Wang et al. 2014). Life expectancy has also increased to 73.8 (compare
to 80.3 years in Western Europe). This improvement in healthcare delivery has resulted
in reduction in communicable diseases and brought noncommunicable diseases including
genetic disorders to the forefront of national healthcare agenda.
Genetic Services in Saudi Arabia
There is more than 30 board certified clinical geneticists in Saudi Arabia, the overwhelming
majority of whom practice in State-funded tertiary centers. Most of these physicians
have received their specialization in clinical genetics abroad but an accredited local
fellowship program in medical genetics has been graduating practicing medical geneticists
since its establishment a few years ago. These physicians cover the major disciplines
of clinical genetics: dysmorphology, inborn errors of metabolism, prenatal and cancer
genetics, and are supported by a limited number of certified genetic counselors. The
overwhelming number of patients with neurocognitive phenotypes compels neurologists,
especially pediatric neurologists, to frequently assume the role of a clinical geneticist
since the average wait-time for clinical geneticists is often >8 months. Similarly,
because thalassemias and hemoglobinopathies are the most frequent Mendelian diseases
in Saudi Arabia (see below), hematologists usually take care of counseling these families
and only refer the most atypical cases to clinical genetics for workup or counseling.
Cytogenetic testing is widely available, usually in the form of traditional karyotyping
and FISH analysis. Molecular karyotyping is only available in a few centers. The major
molecular diagnostic laboratory is at KFSHRC (Saudi Diagnostic Laboratory or SDL),
which tests for 66 single gene disorders. We are currently validating the “Mendeliome”
assay, which uses new multiplexing methods to amplify ∼3000 Mendelian genes known
to cause human diseases followed by next-generation sequencing, on 3500 patients.
Once validated, this test will be available for all patients with suspected genetic
diseases as an intermediary test before considering whole-exome or whole-genome sequencing
(details will be published elsewhere). Whole-exome and whole-genome sequencing are
only available on research basis locally but SDL plans to launch these on clinical
basis in the very near future.
The first Saudi national newborn screening program was for congenital hypothyroidism
and was established in November 1989 (Al-Jurayyan et al. 1996). The pioneering work
of the Tandem Spectrometry Lab at KFSHRC on the use of electrospray in the implementation
of tandem spectrometry in the analysis of various metabolites in body fluids is noteworthy.
It has set the stage for the first implementation of computer-assisted algorithm in
the simultaneous estimation of many metabolites and flagging of abnormal results,
the basis of today's newborn screening around the world (Rashed et al. 1994, 1995,
1997, 1999). Owing to this history, KFSHRC has a long tradition in performing newborn
screening for 16 different inborn errors of metabolism, which evolved into a pilot
program starting in 2004 to screen newborns from participating hospitals around the
country. More recently, the MOH has assumed full responsibility of newborn screening,
which is now performed as a national program. There are no national guidelines on
newborn screening for deafness, which is left to the discretion of the individual
hospitals.
While the newborn screening program was widely accepted, the premarital screening
program was more controversial. After considerable deliberation, a law was passed
in 2002 that mandates screening for hemoglobinopathies, thalassemias, and G6PDH deficiency
prior to issuing a marriage contract. Aside from the controversy surrounding the issue
of autonomy, the program delivered sobering results after its establishment with nearly
90% of “incompatible” couples moving ahead with their marriage plans (the law explicitly
allows couples to exercise freedom of choice upon learning their results) (AlHamdan
et al. 2007). This was clearly the result of inadequate pre- and posttest counseling.
Indeed, major developments in the program to address these deficiencies have significantly
reduced the percentage of “incompatible” marriages to a national average of 40%, with
marked regional variations (large cities such as Riyadh are nearing 20% whereas rural
areas with strong tribal traditions continue to see a majority of “incompatible” couples
moving ahead with marriage) (Memish and Saeedi 2011) (Ayman Alsulaimani, pers. comm.).
There is strong interest in expanding the premarital screening program to include
all Mendelian disorders by utilizing the newly available and affordable next-generation
sequencing tools, and local research is ongoing in order to provide empirical data
on the practicality of this approach.
Prenatal genetics is largely practiced by maternal-fetal medicine specialists due
to severe deficiency in the number of qualified clinical geneticists. Recent years
have witnessed a tremendous growth in the demand for chorionic villous sampling and
amniocentesis for the diagnosis of single gene disorders. At KFSHRC alone, the number
of prenatal samples that are tested for single gene disorders has increased from 5
in 2004 to 250 in 2013. Therapeutic abortion is permitted by law if performed within
120 days from the time of fertilization in order to comply with the Islamic view of
the timing of ensoulment (Alkuraya and Kilani 2001). However, the approved indication
for the procedure, which is “severe malformation”, must be authorized by three attending-level
physicians. The definition of “severe” is left to the discretion of the medical team
after consulting with the family. For example, intellectual disability is a common
indication for many therapeutic abortion procedures. Contrary to commonly held views,
we have shown that early prenatal diagnosis is the method of choice for couples who
had one or more children with single gene disorders, as long as they are provided
with a culturally sensitive genetic counseling that addresses their religious and
cultural concerns (Alkuraya and Kilani 2001). Nearly 45% of these couples opt for
early prenatal diagnosis compared to 35% who choose preimplantation genetic diagnosis
(PGD) (Alkuraya 2013a). PGD is available freely at KFSHRC but is also provided by
the private sector. Noninvasive prenatal screening using cell-free fetal DNA in maternal
blood is quickly becoming integrated in prenatal care. KFSHRC offers this test routinely
to all pregnant women regardless of their perceived risk and the MOH is considering
making this test available throughout its vast network of hospitals and medical centers.
Genetic Disorders in Saudi Arabia
Not surprisingly, the high rate of consanguinity has greatly impacted the landscape
of genetic disorders in Saudi Arabia and a quick search for published genetic diagnoses
from Saudi Arabia readily reveals the clear bias toward autosomal recessive disorders.
There are important practical implications of the role consanguinity plays in shaping
the genetics of Mendelian diseases in Saudi Arabia. For recessive disorders, consanguinity
favors homozygosity over compound heterozygosity, especially for less common conditions,
and this is reflected in the finding that the overwhelming majority of recessive mutations
identified in Saudi diagnostic laboratories are homozygous, a pattern that is echoed
by published studies from Saudi Arabia (Alkuraya 2010a). This phenomenon can easily
be leveraged in the area of diagnostics such that an inexpensive genome-wide homozygosity
scan can greatly aid in the diagnostic work up as shown in detail elsewhere (Alkuraya
2010b). For example, examining the genes within the homozygous intervals can easily
help the clinician to either confirm or reconsider an uncertain clinical diagnosis.
This can also help guide the sequencing effort when a disorder is genetically heterogeneous,
especially when the mutation is not readily detectable, for example, deep intronic,
where prioritizing a particular gene can make more involved tests, for example, RTPCR,
more justifiable. One could argue that this is less relevant now with the availability
of whole-exome sequencing. However, a homozygosity scan can greatly reduce the number
of candidate variants as we have shown in many instances (Alkuraya 2013b). That consanguinity
can render homozygous DNA variants that arose as recently as two generations ago (in
the case of first cousin union) makes it possible for private mutations to be overrepresented
and for allelic heterogeneity to be common as we have shown previously (Aldahmesh
et al. 2009). This has important implications, in that screening approaches that rely
on common mutations are unlikely to be effective in Saudi Arabia, hence the push for
sequencing-based approaches (Kaya et al. 2011). Interestingly, this level of homozygosity
has the potential to reveal unusual patterns of inheritance. In addition to pseuododominance
inheritance, which is seen not infrequently, classical dominant disorders may assume
a recessive pattern of inheritance, for example, we have a case of Treacher-Collins
syndrome caused by a homozygous truncating mutation in TCOF1 while the heterozygous
parents were completely unaffected (unpublished). Alternatively, the same gene that
is known to cause a particular phenotype in the heterozygous state may result in a
novel phenotype in the homozygous state as we have shown for ELOVL4 (Aldahmesh et
al. 2011a).
Similar to the practice of clinical genetics elsewhere, syndromic and nonsyndromic
forms of intellectual disability and developmental delay account for the majority
of referrals to pediatric genetic services in Saudi Arabia. Our unpublished data clearly
show that the majority of these cases have an underlying recessive cause of their
disability, which is in clear contrast to outbred populations where recent studies
on the utility of whole-exome sequencing revealed little or no contribution of recessive
mutations (de Ligt et al. 2012; Rauch et al. 2012).
Many disorders have been first described/mapped in Saudi patients (Table 1). Other
disorders are known to exist elsewhere but are particularly common in Saudi Arabia
(Table 2). For some, this can easily be explained by the disease's high degree of
genetic heterogeneity such that consanguinity can be an important catalyst in unmasking
the recessiveness of numerous potential mutations across many loci, for example, ciliopathies,
retinal dystrophies, and deafness. For others, a strong founder effect can be invoked
as in many inborn errors of metabolism (1.5 in 1000 newborns are diagnosed with a
metabolic disease in the Saudi newborn program) and congenital glaucoma. Geographic
variation in the incidence of diseases has been suggested by some but the mobility
of the population lessens the practical utility of this map especially when one considers
that the geographic variation falls largely along tribal lines, which suggests that
knowledge about the tribal origin can be more helpful clinically (Al-Owain et al.
2012).
Table 1
Clinical conditions first described in Saudi Arabia
Condition
Gene
Reference
Arthrogryposis, Perthes disease, and upward gaze palsy
?
Retinal dystrophy with severe white matter changes
ACBD5
Abu-Safieh et al. (2013)
Weill–Marchesani-like syndrome
ADAMTS17
Morales et al. (2009)
Microcornea, myopic chorioretinal atrophy, and telecanthus (MMCAT)
ADAMTS18
Aldahmesh et al. (2013b)
Intellectual disability-strabismus syndrome
ADAT3
Alazami et al. (2013)
AGK-related cataract
AGK
Aldahmesh et al. (2012a)
Hypopituitarism, microcephaly, and visual and renal anomalies
ARNT2
Webb et al. (2013)
BRCA2-related primordial dwarfism
BRCA2
Shaheen et al. (2014a)
Microphthalmia-dysgenesis of corpus callosum-epilepsy
C12orf57
Zahrani et al. (2013)
C21orf2-related retinal dystrophy
C21orf2
Abu-Safieh et al. (2013)
Woodhouse–Sakati syndrome
C2orf37
Alazami et al. (2008)
Cognitive impairment, dysmorphic facies and skeletal abnormalities syndrome
CACNA1G
Al-Owain et al. (2011)
CENPJ-related Seckel syndrome
CENPJ
Al-Dosari et al. (2010)
Intellectual disability-hypohidrosis syndrome
COG6
Shaheen et al. (2013a)
COLEC11-related Malpuech syndrome
COLEC11
Rooryck et al. (2011)
CRIPT-related primordial dwarfism
CRIPT
Shaheen et al. (2014a)
CSPP1-related Meckel–Gruber syndrome
CSPP1
Shaheen et al. (2014b)
Lethal familial hyperekplexia-brain malformation syndrome
CTSD
Seidahmed et al. (2012)
Myopia with dysmorphism
CTSH
Aldahmesh et al. (2013a)
CYP51A1-related cataract
CYP51A1
Aldahmesh et al. (2012b)
DDX59-related oral-facial-digital syndrome
DDX59
Shamseldin et al. (2013)
DNA2-related Seckel syndrome
DNA2
Shaheen et al. (2014a)
DNASE1L3-related SLE
DNASE1L3
Al-Mayouf et al. (2011)
DOCK6-related Adams-Oliver syndrome
DOCK6
Shaheen et al. (2011a)
Retinal dystrophy with myopathy
DTHD1
Abu-Safieh et al. (2013)
Ichthyosis, spastic quadriplegia, and mental retardation
ELOVL4
Aldahmesh et al. (2011a)
EMC1-related retinal dystrophy
EMC1
Abu-Safieh et al. (2013)
EOGT-related Adams-Oliver syndrome
EOGT
Shaheen et al. (2013b)
Pellagra-like syndrome
ERCC5
Hijazi et al. (2013)
ERLIN2-related complex hereditary spastic paraplegia
ERLIN2
Alazami et al. (2011)
EVC2-related Meckel–Gruber syndrome
EVC2
Shaheen et al. (2012a)
FARS2-related mitochondrial encephalomyopathy
FARS2
Shamseldin et al. (2012a)
FBXL4-related mitochondrial encephalomyopathy
FBXL4
Gai et al. (2013)
Bruck syndrome 1
FKBP10
Shaheen et al. (2010)
G6PC3-related cyclic neutropenia
G6PC3
Alangari et al. (2013)
GPR125-related retinal dystrophy
GPR125
Abu-Safieh et al. (2013)
IFT27-related Bardet–Biedl syndrome
IFT27
Aldahmesh et al. (2014)
Familial retinal artery macroaneurysm
IGFBP7
Abu-Safieh et al. (2011)
Congenital hyperinsulinemia with rhabdomyolysis
KCNJ11
Albaqumi et al. (2014)
KIAA1549-related retinal dystrophy
KIAA1549
Abu-Safieh et al. (2013)
KLHL41-related myopathy
KLHL41
Gupta et al. (2013)
Facial dysmorphism with severe growth deficiency
LARP7
Alazami et al. (2012)
LRBA-related Crohn's disease with immunodeficiency
LRBA
Alangari et al. (2012)
LRPAP1-related myopia
LRPAP1
Aldahmesh et al. (2013a)
MEOX1-related Klippel–Feil syndrome
MEOX1
Mohamed et al. (2013)
METTL23-related intellectual disability
METTL23
Reiff et al. (2014)
MFF-related mitochondrial encephalomyopathy
MFF
Shamseldin et al. (2012a)
MMP2-related multicentric osteolysis
MMP2
Al-Aqeel (2005)
MPDZ-related hydrocephalus
MPDZ
Al-Dosari et al. (2013)
MRI1-related infantile epilepsy with severe cystic degeneration of the brain
MRI1
Sunker and Alkuraya (2013)
Bone marrow failure with facial dysmorphsim
MYSM1
Alsultan et al. (2013)
NECAP1-related early infantile epileptic encephalopathy
NECAP1
Alazami et al. (2014a)
ODZ3-related microphthalmia
ODZ3
Aldahmesh et al. (2012c)
OPLAH-related oxoprolinurai
OPLAH
Almaghlouth et al. (2012)
PHC1-related microcephaly
PHC1
Awad et al. (2013)
PHGDH-related Neu-Laxova syndrome
PHGDH
Shaheen et al. (2014c)
PITX3-related microphthalmia
PITX3
Aldahmesh et al. (2011b)
POC1A-related primordial dwarfism
POC1A
Shaheen et al. (2012b)
RAB33B-related Smith–McCort dysplasia
RAB33B
Alshammari et al. (2012)
CMT-microcephaly-syndactyly-intellectual disability
SBF1
Alazami et al. (2014b))
SCLT1-related oral-facial-digital syndrome
SCLT1
Adly et al. (2014)
SEC8-related Meckel–Gruber syndrome
SEC8
Shaheen et al. (2012a)
SIX6-related autosomal recessive microphthalmia
SIX6
Aldahmesh et al. (2013c)
TBC1D32-related oral-facial-digital syndrome
TBC1D32
Adly et al. (2014)
Congenital hypoparathyroidism, severe growth failure, and dysmorphic facies
TBCE
Sanjad et al. (1991)
TCTN2-related Meckel–Gruber syndrome
TCTN2
Shaheen et al. (2011b)
TMEM231-related Meckel–Gruber syndrome
TMEM231
Shaheen et al. (2013c)
TMEM38-related osteogenesis imperfecta
TMEM38B
Shaheen et al. (2012c)
Osteogenesis imperfecta with profound neurological impairment
WNT1
Faqeih et al. (2013)
XRCC2-related Fanconi anemia
XRCC2
Shamseldin et al. (2012b)
XRCC4-related primordial dwarfism
XRCC4
Shaheen et al. (2014a)
Table 2
Frequently encountered Mendelian conditions in Saudi Arabia
Sickel-cell anemia
Thalassemia
Intellectual disability
Congenital glaucoma
Bardet–Biedl syndrome
Meckel–Gruber syndrome
Organic acidemias
Lysosomal storage disorders
Retinal dystrophies
Hearing loss
Primary microcephaly
Opportunities in Genomic Medicine in Saudi Arabia
The high rate of consanguinity in Saudi Arabia has long been exploited to accelerate
the annotation of recessive Mendelian genes and the recent years have witnessed a
marked shift towards building infrastructure that permits this line of research to
be performed locally. This trend has made a positive impact on the attitude of young
Saudis to pursue careers in human genetics. But the study of rare recessive Mendelian
disorders is only one of many opportunities that genomic research in Saudi Arabia
has to offer. For example, identification of Mendelian forms of common diseases can
provide novel insights into pathogenic mechanisms that could prove relevant to the
common forms of these diseases (Al-Mayouf et al. 2011; Alangari et al. 2012; Aldahmesh
et al. 2013a). Beyond Mendelian disorders, genomic analysis of Saudis has proved to
be a valuable resource to track nullizygous DNA segments and biallelically inactivated
genes in nondiseased individuals (Khalak et al. 2012). Not only does this line of
research have the potential to improve the annotation of the human genome in terms
of its clinical relevance, but it can also identify novel druggable targets by identifying
genes whose loss of function brings about desirable phenotypic traits as recently
shown with PCSK9 and CCR5 (Lederman et al. 2006; Rader and Daugherty 2008). In addition,
the lack of representation of Saudi genomes in international GWAS consortia presents
an opportunity to identify potentially novel risk alleles for common diseases as shown
recently with the identification of a novel risk allele for complications of HBV infection
(Al-Qahtani et al. 2013). A very recent study has shown the potential of genetically
isolated societies to reveal novel risk alleles using a fraction of the usual study
cohort size for a typical GWAS (Moltke et al. 2014), and this should provide an additional
impetus to explore the genetics of common diseases among Saudis.
In recognition of these opportunities, the Saudi Government has recently announced
its plan to fund the sequencing of 100,000 Saudis as part of the newly launched Saudi
Human Genome Project. The above lines of research and others will form the basis of
selecting the 100,000 Saudis to be sequenced. For example, 10,000 healthy Saudis will
have their genomes sequenced specifically in search of biallelically inactivated genes
(Kaiser 2014).
It is clear that Saudi Arabia has been and will continue to be an important resource
in the study of Mendelian genes, and recent technological advances are diversifying
the relevance of this resource to the various fields of genomic medicine. The time
has never been more opportune for conducting genomic research in Saudi Arabia to empower
Saudis to reap its promise of better health.
Related collections
Most cited references71
- Record: found
- Abstract: found
- Article: found
Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013
- Record: found
- Abstract: found
- Article: not found
Translating molecular discoveries into new therapies for atherosclerosis.
Daniel Rader, Alan Daugherty (2008)

- Record: found
- Abstract: found
- Article: found
Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes
Leen Abu-Safieh, May Alrashed, Shamsa Anazi … (2013)