- Record: found
- Abstract: found
- Article: found
Platinum-modified covalent triazine frameworks hybridized with carbon nanoparticles as methanol-tolerant oxygen reduction electrocatalysts

Read this article at
Abstract
Covalent triazine frameworks, which are crosslinked porous polymers with two-dimensional molecular structures, are promising materials for heterogeneous catalysts. However, the application of the frameworks as electrocatalysts has not been achieved to date because of their poor electrical conductivity. Here we report that platinum-modified covalent triazine frameworks hybridized with conductive carbon nanoparticles are successfully synthesized by introducing carbon nanoparticles during the polymerization process of covalent triazine frameworks. The resulting materials exhibit clear electrocatalytic activity for oxygen reduction reactions in acidic solutions. More interestingly, the platinum-modified covalent triazine frameworks show almost no activity for methanol oxidation, in contrast to commercial carbon-supported platinum. Thus, platinum-modified covalent triazine frameworks hybridized with carbon nanoparticles exhibit selective activity for oxygen reduction reactions even in the presence of high concentrations of methanol, which indicates potential utility as a cathode catalyst in direct methanol fuel cells.
Abstract
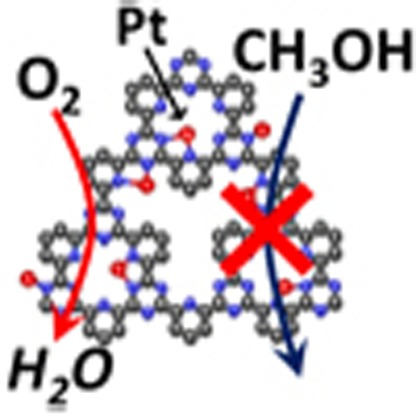 Covalent triazine frameworks are known catalysts for some catalytic reactions, but
show no electrocatalytic activity. Here, the authors synthesize platinum modified
covalent triazine frameworks hybridized with carbon nanoparticles, which are electro-active
for oxygen reduction reactions.
Covalent triazine frameworks are known catalysts for some catalytic reactions, but
show no electrocatalytic activity. Here, the authors synthesize platinum modified
covalent triazine frameworks hybridized with carbon nanoparticles, which are electro-active
for oxygen reduction reactions.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Identification of active gold nanoclusters on iron oxide supports for CO oxidation.
- Record: found
- Abstract: found
- Article: not found
Platinum catalysts for the high-yield oxidation of methane to a methanol derivative
- Record: found
- Abstract: found
- Article: not found