- Record: found
- Abstract: found
- Article: found
Shenfu Injection attenuates rat myocardial hypertrophy by up-regulating miR-19a-3p expression
Read this article at
Abstract
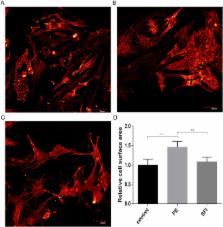
Shenfu Injection (SFI) is a classical Chinese medicine used to treat heart failure. Our previous study demonstrated that miRNAs underwent changes in rats with myocardial hypertrophy induced by abdominal aortic constriction. Interestingly, there was a significant change in miR-19a-3p, whose target gene is known to be associated with MEF2 signaling. However, whether and how SFI regulates miR-19a-3p in the treatment of myocardial hypertrophy has not been investigated. The purpose of the present study was to investigate the regulatory effect of SFI on miR-19a-3p in MEF2 signaling in the rat hypertrophic myocardium. We found that the miR-19a-3p expression level was significantly decreased in the hypertrophic myocardium, and MEF2A was the target gene of miR-19a-3p. The protein expressions of MEF2A, β-MHC, BNP and TRPC1 were significantly increased in hypertrophic cardiomyocytes. MiR-19a-3p was up-regulated after SFI treatment, and the protein expressions of these genes were significantly decreased. In addition, miR-19a-3p over-expression in hypertrophic cardiomyocytes could decrease MEF2A mRNA and protein expressions, and anti-miR-19a-3p showed the opposite result. Our study provided substantial evidence that miR-19a-3p played a functional role in MEF2 signaling in myocardial hypertrophy. SFI attenuated cardiomyocyte hypertrophy probably through up-regulating or maintaining the miR-19a-3p levels and regulating the MEF2 signaling pathway.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice.
- Record: found
- Abstract: found
- Article: not found
Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies.
- Record: found
- Abstract: found
- Article: not found