- Record: found
- Abstract: found
- Article: found
Transient Cannabinoid Receptor 2 Blockade during Immunization Heightens Intensity and Breadth of Antigen-specific Antibody Responses in Young and Aged mice
Read this article at
Abstract
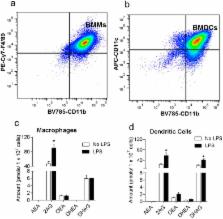
The hallmark of vaccines is their ability to prevent the spread of infectious pathogens and thereby serve as invaluable public health tool. Despite their medical relevance, there is a gap in our understanding of the physiological factors that mediate innate and adaptive immune response to vaccines. The endocannabinoid (eCB) system is a critical modulator of homeostasis in vertebrates. Our results indicate that macrophages and dendritic cells produce the endocannabinoid, 2-arachidonoyl-sn-glycerol (2-AG) upon antigen activation. We have also established that 2-AG levels are upregulated in the serum and in the lymph node of mice during vaccination. We hypothesized that the intrinsic release of eCBs from immune cells during activation by pathogenic antigens mitigate inflammation, but also suppress overall innate and adaptive immune response. Here we demonstrate, for the first time, that transient administration of the cannabinoid receptor 2 antagonist AM630 (10 mg/kg) or inverse agonist JTE907 (3 mg/kg) during immunization heightens the intensity and breadth of antigen-specific immune responses in young and aged mice through the upregulation of immunomodulatory genes in secondary lymphoid tissues.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations.
- Record: found
- Abstract: found
- Article: not found
Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation.
- Record: found
- Abstract: found
- Article: not found