- Record: found
- Abstract: found
- Article: found
Molecular spectrum of TSHβ subunit gene defects in central hypothyroidism in the UK and Ireland
Summary
Objective
Homozygous mutations in the TSH beta subunit gene ( TSHB ) result in severe, isolated, central congenital hypothyroidism ( CCH). This entity evades diagnosis in TSH‐based congenital hypothyroidism ( CH) screening programmes in the UK and Ireland. Accordingly, genetic diagnosis, enabling ascertainment of affected relatives in families, is critical for prompt diagnosis and treatment of the disorder.
Design, Patients and Measurements
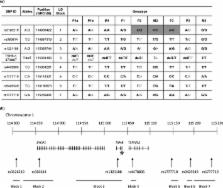
Four cases of isolated TSH deficiency from three unrelated families in the UK and Ireland were investigated for mutations or deletions in TSHB . Haplotype analysis, to investigate a founder effect, was undertaken in cases with identical mutations (c.373delT).
Results
Two siblings in kindred 1 were homozygous for a previously described TSHB mutation (c.373delT). In kindreds 2 and 3, the affected individuals were compound heterozygous for TSHB c.373delT and either a 5·4‐ kB TSHB deletion (kindred 2, c.1‐4389_417*195delins CTCA) or a novel TSHB missense mutation (kindred 3, c.2T>C, p.Met1?). Neurodevelopmental retardation, following delayed diagnosis and treatment, was present in 3 cases. In contrast, the younger sibling in kindred 1 developed normally following genetic diagnosis and treatment from birth.
Conclusions
This study, including the identification of a second, novel, TSHB deletion, expands the molecular spectrum of TSHB defects and suggests that allele loss may be a commoner basis for TSH deficiency than previously suspected. Delayed diagnosis and treatment of profound central hypothyroidism in such cases result in neurodevelopmental retardation. Inclusion of thyroxine (T4) plus thyroxine‐binding globulin ( TBG), or free thyroxine ( FT4) in CH screening, together with genetic case ascertainment enabling earlier therapeutic intervention, could prevent such adverse sequelae.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings.
- Record: found
- Abstract: found
- Article: not found
Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships.
- Record: found
- Abstract: found
- Article: not found