- Record: found
- Abstract: found
- Article: found
Route, mechanism, and implications of proton import during Na +/K + exchange by native Na +/K +-ATPase pumps
Read this article at
Abstract
The Na +/K + pump is a hybrid transporter that can also import protons at physiological K + and Na + concentrations.
Abstract
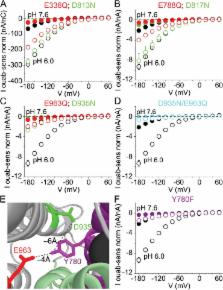
A single Na +/K +-ATPase pumps three Na + outwards and two K + inwards by alternately exposing ion-binding sites to opposite sides of the membrane in a conformational sequence coupled to pump autophosphorylation from ATP and auto-dephosphorylation. The larger flow of Na + than K + generates outward current across the cell membrane. Less well understood is the ability of Na +/K + pumps to generate an inward current of protons. Originally noted in pumps deprived of external K + and Na + ions, as inward current at negative membrane potentials that becomes amplified when external pH is lowered, this proton current is generally viewed as an artifact of those unnatural conditions. We demonstrate here that this inward current also flows at physiological K + and Na + concentrations. We show that protons exploit ready reversibility of conformational changes associated with extracellular Na + release from phosphorylated Na +/K + pumps. Reversal of a subset of these transitions allows an extracellular proton to bind an acidic side chain and to be subsequently released to the cytoplasm. This back-step of phosphorylated Na +/K + pumps that enables proton import is not required for completion of the 3 Na +/2 K + transport cycle. However, the back-step occurs readily during Na +/K + transport when external K + ion binding and occlusion are delayed, and it occurs more frequently when lowered extracellular pH raises the probability of protonation of the externally accessible carboxylate side chain. The proton route passes through the Na +-selective binding site III and is distinct from the principal pathway traversed by the majority of transported Na + and K + ions that passes through binding site II. The inferred occurrence of Na +/K + exchange and H + import during the same conformational cycle of a single molecule identifies the Na +/K + pump as a hybrid transporter. Whether Na +/K + pump–mediated proton inflow may have any physiological or pathophysiological significance remains to be clarified.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
Voltage-gated proton channels and other proton transfer pathways.
- Record: found
- Abstract: found
- Article: not found
Glutamate transporters: confining runaway excitation by shaping synaptic transmission.
- Record: found
- Abstract: found
- Article: not found