- Record: found
- Abstract: found
- Article: not found
Global aspects of viral glycosylation
Read this article at
Abstract
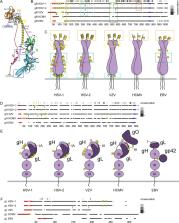
Enveloped viruses encompass some of the most common human pathogens causing infections of different severity, ranging from no or very few symptoms to lethal disease as seen with the viral hemorrhagic fevers. All enveloped viruses possess an envelope membrane derived from the host cell, modified with often heavily glycosylated virally encoded glycoproteins important for infectivity, viral particle formation and immune evasion. While N-linked glycosylation of viral envelope proteins is well characterized with respect to location, structure and site occupancy, information on mucin-type O-glycosylation of these proteins is less comprehensive. Studies on viral glycosylation are often limited to analysis of recombinant proteins that in most cases are produced in cell lines with a glycosylation capacity different from the capacity of the host cells. The glycosylation pattern of the produced recombinant glycoproteins might therefore be different from the pattern on native viral proteins. In this review, we provide a historical perspective on analysis of viral glycosylation, and summarize known roles of glycans in the biology of enveloped human viruses. In addition, we describe how to overcome the analytical limitations by using a global approach based on mass spectrometry to identify viral O-glycosylation in virus-infected cell lysates using the complex enveloped virus herpes simplex virus type 1 as a model. We underscore that glycans often pay important contributions to overall protein structure, function and immune recognition, and that glycans represent a crucial determinant for vaccine design. High throughput analysis of glycosylation on relevant glycoprotein formulations, as well as data compilation and sharing is therefore important to identify consensus glycosylation patterns for translational applications.
Related collections
Most cited references308
- Record: found
- Abstract: not found
- Article: not found
Assembly of asparagine-linked oligosaccharides.
- Record: found
- Abstract: found
- Article: not found
Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family.
- Record: found
- Abstract: found
- Article: not found
