- Record: found
- Abstract: found
- Article: found
Biomonitoring of Lead, Cadmium, Total Mercury, and Methylmercury Levels in Maternal Blood and in Umbilical Cord Blood at Birth in South Korea
Read this article at
Abstract
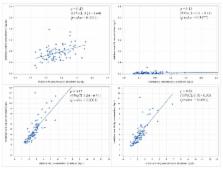
With rising concerns of heavy metal exposure in pregnancy and early childhood, this study was conducted to assess the relationship between the lead, cadmium, mercury, and methylmercury blood levels in pregnancy and neonatal period. The study population included 104 mothers and their children pairs who completed both baseline maternal blood sampling at the second trimester and umbilical cord blood sampling at birth. The geometric mean maternal blood levels of lead, cadmium, total mercury, and methylmercury at the second trimester were 1.02 ± 1.39 µg/dL, 0.61 ± 1.51 µg/L, 2.97 ± 1.45 µg/L, and 2.39 ± 1.45 µg/L, respectively, and in the newborns, these levels at birth were 0.71 ± 1.42 µg/dL, 0.01 ± 5.31 µg/L, 4.44 ± 1.49 µg/L, and 3.67 ± 1.51 µg/L, respectively. The mean ratios of lead, cadmium, total mercury, and methylmercury levels in the newborns to those in the mothers were 0.72, 0.04, 1.76, and 1.81, respectively. The levels of most heavy metals in pregnant women and infants were higher in this study than in studies from industrialized western countries. The placenta appears to protect fetuses from cadmium; however, total mercury and methylmercury were able to cross the placenta and accumulate in fetuses.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Neurotoxic effects and biomarkers of lead exposure: a review.
- Record: found
- Abstract: found
- Article: not found
The role of the placenta in fetal exposure to heavy metals.
- Record: found
- Abstract: found
- Article: not found