- Record: found
- Abstract: found
- Article: found
Integral Characterization of Defective BDNF/TrkB Signalling in Neurological and Psychiatric Disorders Leads the Way to New Therapies
Read this article at
Abstract
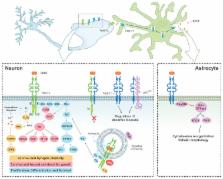
Enhancement of brain-derived neurotrophic factor (BDNF) signalling has great potential in therapy for neurological and psychiatric disorders. This neurotrophin not only attenuates cell death but also promotes neuronal plasticity and function. However, an important challenge to this approach is the persistence of aberrant neurotrophic signalling due to a defective function of the BDNF high-affinity receptor, tropomyosin-related kinase B (TrkB), or downstream effectors. Such changes have been already described in several disorders, but their importance as pathological mechanisms has been frequently underestimated. This review highlights the relevance of an integrative characterization of aberrant BDNF/TrkB pathways for the rational design of therapies that by combining BDNF and TrkB targets could efficiently promote neurotrophic signalling.
Related collections
Most cited references177
- Record: found
- Abstract: found
- Article: not found
A neurotrophic model for stress-related mood disorders.
- Record: found
- Abstract: found
- Article: not found
NMDA Receptor Blockade at Rest Triggers Rapid Behavioural Antidepressant Responses
- Record: found
- Abstract: found
- Article: not found