- Record: found
- Abstract: found
- Article: found
Mesenchymal Stromal Cell-Based Therapies as Promising Treatments for Muscle Regeneration After Snakebite Envenoming
Read this article at
Abstract
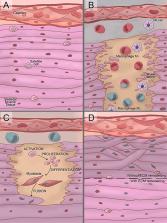
Snakebite envenoming is a global neglected disease with an incidence of up to 2.7 million new cases every year. Although antivenoms are so-far the most effective treatment to reverse the acute systemic effects induced by snakebite envenoming, they have a limited therapeutic potential, being unable to completely neutralize the local venom effects. Local damage, such as dermonecrosis and myonecrosis, can lead to permanent sequelae with physical, social, and psychological implications. The strong inflammatory process induced by snake venoms is associated with poor tissue regeneration, in particular the lack of or reduced skeletal muscle regeneration. Mesenchymal stromal cells (MSCs)-based therapies have shown both anti-inflammatory and pro-regenerative properties. We postulate that using allogeneic MSCs or their cell-free products can induce skeletal muscle regeneration in snakebite victims, improving all the three steps of the skeletal muscle regeneration process, mainly by anti-inflammatory activity, paracrine effects, neovascularization induction, and inhibition of tissue damage, instrumental for microenvironment remodeling and regeneration. Since snakebite envenoming occurs mainly in areas with poor healthcare, we enlist the principles and potential of MSCs-based therapies and discuss regulatory issues, good manufacturing practices, transportation, storage, and related-procedures that could allow the administration of these therapies, looking forward to a safe and cost-effective treatment for a so far unsolved and neglected health problem.
Related collections
Most cited references128
- Record: found
- Abstract: found
- Article: not found
Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement.
- Record: found
- Abstract: found
- Article: not found
Multilineage potential of adult human mesenchymal stem cells.
- Record: found
- Abstract: found
- Article: not found